Abstract
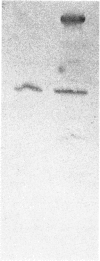
Rat uteri were taken at various stages of pregnancy and involution post partum, and several other tissues were taken from pregnant and non-pregnant animals. Portions of each tissue were homogenized in the presence of proteinase inhibitors, and the amounts of the high-Ca2+-requiring Ca2+-activated proteinase in the supernatants were measured by a two-site immunoradiometric assay using 125I-immunoglobulin G. The proteinase was shown, by protein blotting, to be immunologically identical in all tissues. The amounts in the various tissues, expressed in units of proteinase activity/g wet wt., were: lung, 95; kidney and small intestine, 42; liver, 20; brain, heart and skeletal muscle, 13. Uterine wet weight increased at the end of pregnancy by about 8-fold, but the amounts of proteinase per uterus increased by about 22-fold; alternatively, expressed in units of proteinase activity/g wet wt., the mean uterine values were: non-pregnant, 28.6; term-pregnant, 77.0. As the wet weight of the uterus fell rapidly during involution, the amounts of proteinase activity remained relatively high. The data suggest that the Ca2+-activated proteinase may have some role in tissue resorption during uterine involution, but the high proteinase activity present before parturition must be regulated in ways which are not yet clear.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afting E. G., Becker M. L., Elce J. S. Proteinase and proteinase-inhibitor activities of rat uterine myometrium during pregnancy and involution. Biochem J. 1979 Jan 1;177(1):99–106. doi: 10.1042/bj1770099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afting E. G., Elce J. S. DNA in the rat uterus myometrium during pregnancy and postpartum involution. Measurement of DNA in small pieces of mammalian tissue. Anal Biochem. 1978 May;86(1):90–99. doi: 10.1016/0003-2697(78)90321-4. [DOI] [PubMed] [Google Scholar]
- Ahkong Q. F., Botham G. M., Woodward A. W., Lucy J. A. Calcium-activated thiol-proteinase activity in the fusion of rat erythrocytes induced by benzyl alcohol. Biochem J. 1980 Dec 15;192(3):829–836. doi: 10.1042/bj1920829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azanza J. L., Cottin P., Robin J. M., Raymond J., Ducastaing A. Calcium-ion-activated neutral proteinase of muscle is found in other tissues. Biochimie. 1981 Aug-Sep;63(8-9):729–732. doi: 10.1016/s0300-9084(81)80222-2. [DOI] [PubMed] [Google Scholar]
- Azanza J. L., Raymond J., Robin J. M., Cottin P., Ducastaing A. Purification and some physico-chemical and enzymic properties of a calcium ion-activated neutral proteinase from rabbit skeletal muscle. Biochem J. 1979 Nov 1;183(2):339–347. doi: 10.1042/bj1830339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth R., Elce J. S. Immunofluorescent localization of a Ca2+-dependent neutral protease in hamster muscle. Am J Physiol. 1981 May;240(5):E493–E498. doi: 10.1152/ajpendo.1981.240.5.E493. [DOI] [PubMed] [Google Scholar]
- Baudry M., Lynch G. Regulation of hippocampal glutamate receptors: evidence for the involvement of a calcium-activated protease. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2298–2302. doi: 10.1073/pnas.77.4.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R. D., Cumming R. Evidence for the presence of high-Mr microtubule-associated proteins and their Ca2+-dependent proteolysis in synaptosomal cytosol. FEBS Lett. 1982 Sep 20;146(2):273–277. doi: 10.1016/0014-5793(82)80933-2. [DOI] [PubMed] [Google Scholar]
- Croall D. E., DeMartino G. N. Purification and characterization of calcium-dependent proteases from rat heart. J Biol Chem. 1983 May 10;258(9):5660–5665. [PubMed] [Google Scholar]
- Dayton W. R. Comparison of low- and high-calcium-requiring forms of the calcium-activated protease with their autocatalytic breakdown products. Biochim Biophys Acta. 1982 Dec 20;709(2):166–172. doi: 10.1016/0167-4838(82)90457-5. [DOI] [PubMed] [Google Scholar]
- Dayton W. R., Reville W. J., Goll D. E., Stromer M. H. A Ca2+-activated protease possibly involved in myofibrillar protein turnover. Partial characterization of the purified enzyme. Biochemistry. 1976 May 18;15(10):2159–2167. doi: 10.1021/bi00655a020. [DOI] [PubMed] [Google Scholar]
- Elce J. S., Hasspieler R., Boegman R. J. Ca2+-activated protease in denervated rat skeletal muscle measured by an immunoassay. Exp Neurol. 1983 Aug;81(2):320–329. doi: 10.1016/0014-4886(83)90266-2. [DOI] [PubMed] [Google Scholar]
- Etherington D. J. Collagenolytic-cathepsin and acid-proteinase activities in the rat uterus during post partum involution. Eur J Biochem. 1973 Jan 3;32(1):126–128. doi: 10.1111/j.1432-1033.1973.tb02587.x. [DOI] [PubMed] [Google Scholar]
- Fox J. E., Reynolds C. C., Phillips D. R. Calcium-dependent proteolysis occurs during platelet aggregation. J Biol Chem. 1983 Aug 25;258(16):9973–9981. [PubMed] [Google Scholar]
- Gerard K. W., Schneider D. L. Protein turnover in muscle: inhibitions of the calcium activated proteinase by mersalyl without inhibition of the rate of protein degradation. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1353–1361. doi: 10.1016/0006-291x(80)90568-9. [DOI] [PubMed] [Google Scholar]
- Gershoni J. M., Palade G. E. Protein blotting: principles and applications. Anal Biochem. 1983 May;131(1):1–15. doi: 10.1016/0003-2697(83)90128-8. [DOI] [PubMed] [Google Scholar]
- Hales C. N., Woodhead J. S. Labeled antibodies and their use in the immunoradiometric assay. Methods Enzymol. 1980;70(A):334–355. doi: 10.1016/s0076-6879(80)70063-0. [DOI] [PubMed] [Google Scholar]
- Ishiura S., Murofushi H., Suzuki K., Imahori K. Studies of a calcium-activated neutral protease from chicken skeletal muscle. I. Purification and characterization. J Biochem. 1978 Jul;84(1):225–230. doi: 10.1093/oxfordjournals.jbchem.a132111. [DOI] [PubMed] [Google Scholar]
- Ishiura S., Sugita H., Nonaka I., Imahori K. Calcium-activated neutral protease. Its localization in the myofibril, especially at the Z-band. J Biochem. 1980 Jan;87(1):343–346. doi: 10.1093/oxfordjournals.jbchem.a132743. [DOI] [PubMed] [Google Scholar]
- Ishizaki Y., Tashiro T., Kurokawa M. A calcium-activated protease which preferentially degrades the 160-kDa component of the neurofilament triplet. Eur J Biochem. 1983 Mar 1;131(1):41–45. doi: 10.1111/j.1432-1033.1983.tb07229.x. [DOI] [PubMed] [Google Scholar]
- Kar N. C., Pearson C. M. Fructose 1,6-diphosphatase in normal and diseased human muscle. Clin Chim Acta. 1972 Apr;38(1):252–254. doi: 10.1016/0009-8981(72)90239-2. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. Radioiodination of proteins by the use of the chloramine-T method. Methods Enzymol. 1980;70(A):210–213. doi: 10.1016/s0076-6879(80)70050-2. [DOI] [PubMed] [Google Scholar]
- Mellgren R. L., Repetti A., Muck T. C., Easly J. Rabbit skeletal muscle calcium-dependent protease requiring millimolar CA2+. Purification, subunit structure, and Ca2+-dependent autoproteolysis. J Biol Chem. 1982 Jun 25;257(12):7203–7209. [PubMed] [Google Scholar]
- Olmsted J. B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [PubMed] [Google Scholar]
- Parakkal P. F. Involvement of macrophages in collagen resorption. J Cell Biol. 1969 Apr;41(1):345–354. doi: 10.1083/jcb.41.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. R., Jakábová M. Ca2+-dependent protease in human platelets. Specific cleavage of platelet polypeptides in the presence of added Ca2+. J Biol Chem. 1977 Aug 25;252(16):5602–5605. [PubMed] [Google Scholar]
- Puca G. A., Nola E., Sica V., Bresciani F. Estrogen binding proteins of calf uterus. Molecular and functional characterization of the receptor transforming factor: A Ca2+-activated protease. J Biol Chem. 1977 Feb 25;252(4):1358–1366. [PubMed] [Google Scholar]
- Reddy M. K., Etlinger J. D., Rabinowitz M., Fischman D. A., Zak R. Removal of Z-lines and alpha-actinin from isolated myofibrils by a calcium-activated neutral protease. J Biol Chem. 1975 Jun 10;250(11):4278–4284. [PubMed] [Google Scholar]
- Rodemann H. P., Waxman L., Goldberg A. L. The stimulation of protein degradation in muscle by Ca2+ is mediated by prostaglandin E2 and does not require the calcium-activated protease. J Biol Chem. 1982 Aug 10;257(15):8716–8723. [PubMed] [Google Scholar]
- Sasaki T., Yoshimura N., Kikuchi T., Hatanaka M., Kitahara A., Sakihama T., Murachi T. Similarity and dissimilarity in subunit structures of calpains I and II from various sources as demonstrated by immunological cross-reactivity. J Biochem. 1983 Dec;94(6):2055–2061. doi: 10.1093/oxfordjournals.jbchem.a134561. [DOI] [PubMed] [Google Scholar]
- Sellers A., Woessner J. F., Jr The extraction of a neutral metalloproteinase from the involuting rat uterus, and its action on cartilage proteoglycan. Biochem J. 1980 Sep 1;189(3):521–531. doi: 10.1042/bj1890521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Tsuji S., Ishiura S., Kimura Y., Kubota S., Imahori K. Autolysis of calcium-activated neutral protease of chicken skeletal muscle. J Biochem. 1981 Dec;90(6):1787–1793. doi: 10.1093/oxfordjournals.jbchem.a133656. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truglia J. A., Stracher A. Purification and characterization of a calcium dependent sulfhydryl protease from human platelets. Biochem Biophys Res Commun. 1981 May 29;100(2):814–822. doi: 10.1016/s0006-291x(81)80247-1. [DOI] [PubMed] [Google Scholar]
- Vedeckis W. V., Freeman M. R., Schrader W. T., O'Malley B. W. Progesterone-binding components of chick oviduct: partial purification and characterization of a calcium-activated protease which hydrolyzes the progesterone receptor. Biochemistry. 1980 Jan 22;19(2):335–343. doi: 10.1021/bi00543a014. [DOI] [PubMed] [Google Scholar]
- Waxman L. Calcium-activated proteases in mammalian tissues. Methods Enzymol. 1981;80(Pt 100):664–680. doi: 10.1016/s0076-6879(81)80051-1. [DOI] [PubMed] [Google Scholar]
- Wheelock M. J. Evidence for two structurally different forms of skeletal muscle Ca2+-activated protease. J Biol Chem. 1982 Nov 10;257(21):12471–12474. [PubMed] [Google Scholar]
- Woessner J. F., Jr Acid hydrolases of the rat uterus in relation to pregnancy, post-partum involution and collagen breakdown. Biochem J. 1965 Dec;97(3):855–866. doi: 10.1042/bj0970855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N., Kikuchi T., Sasaki T., Kitahara A., Hatanaka M., Murachi T. Two distinct Ca2+ proteases (calpain I and calpain II) purified concurrently by the same method from rat kidney. J Biol Chem. 1983 Jul 25;258(14):8883–8889. [PubMed] [Google Scholar]