Abstract
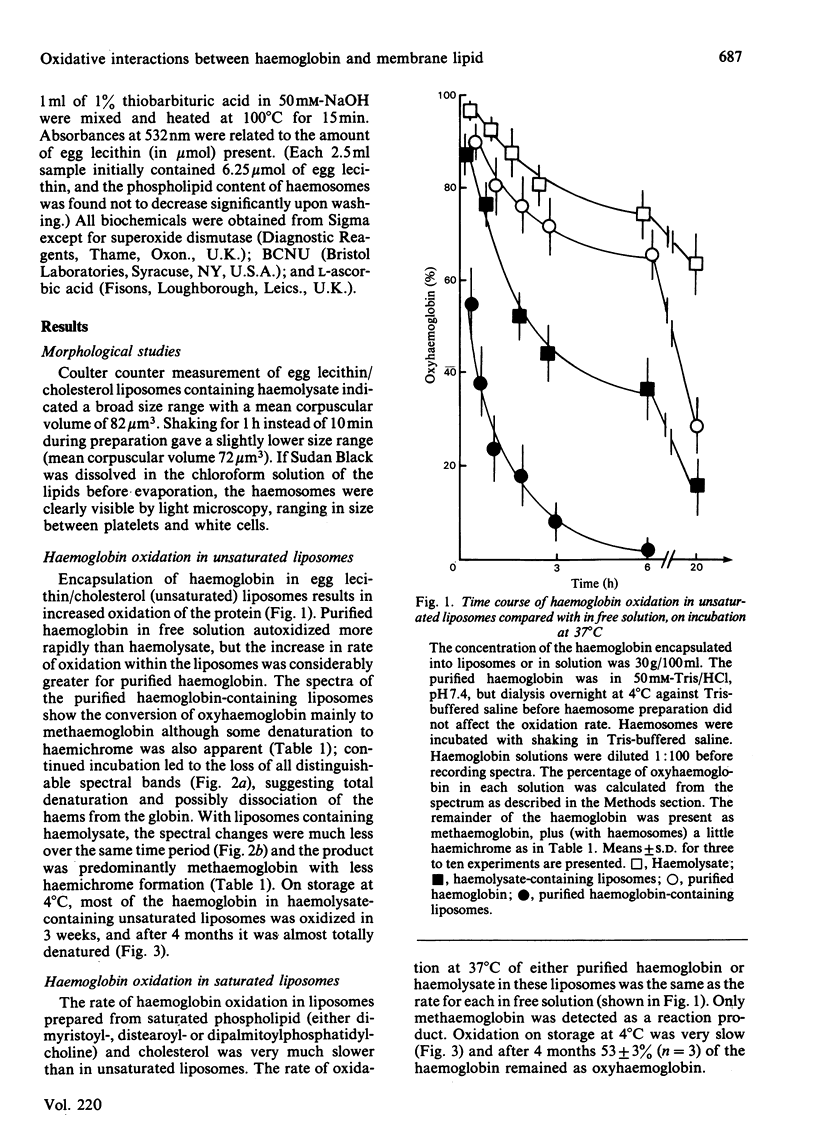
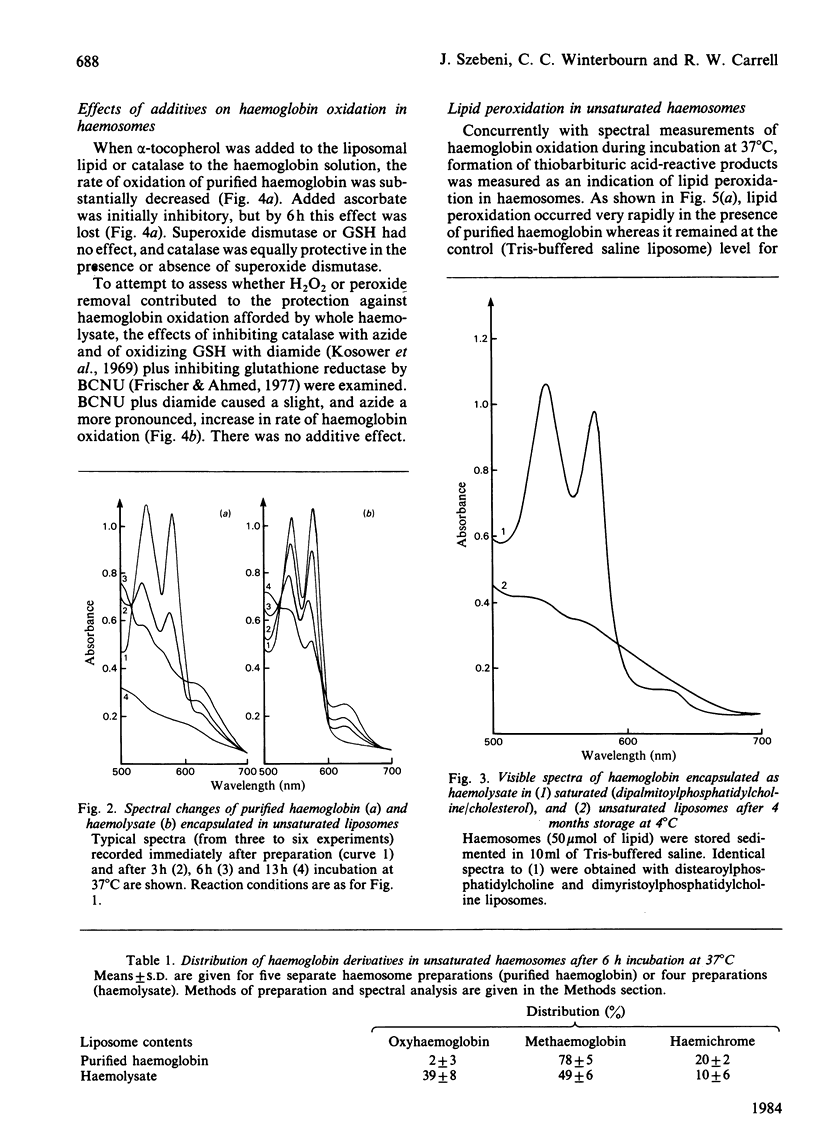
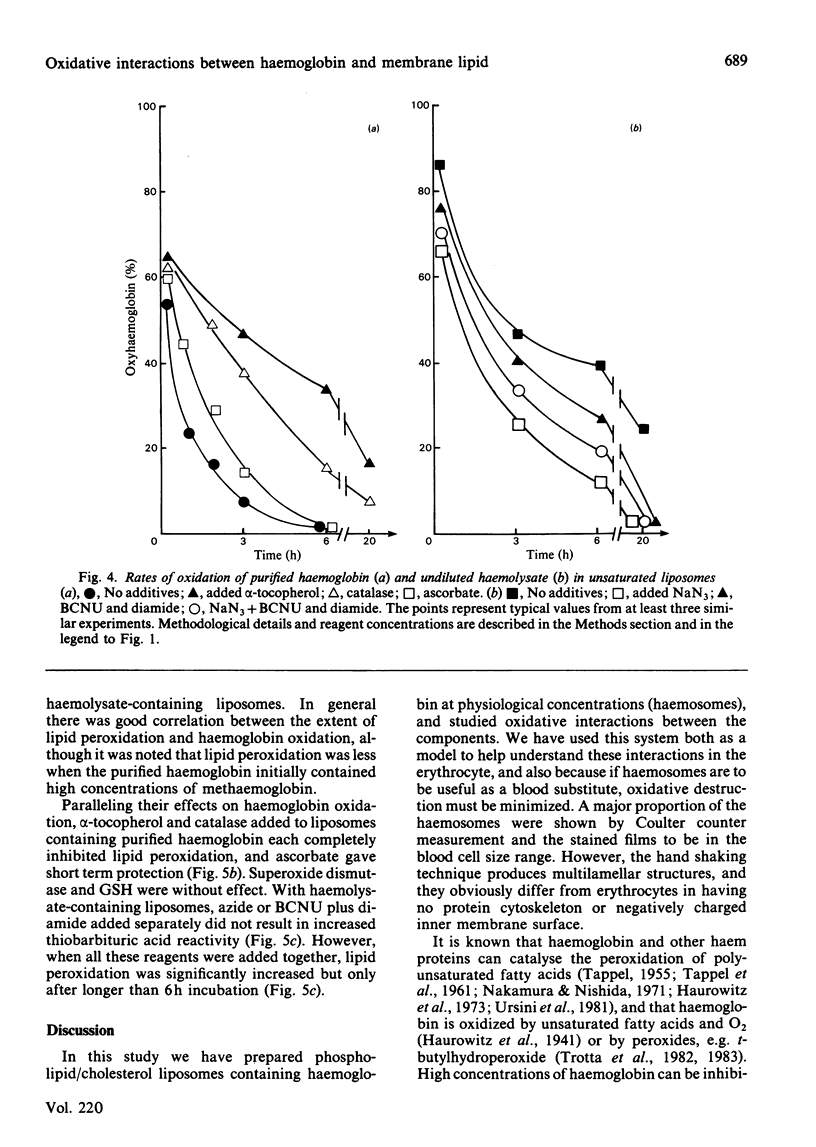
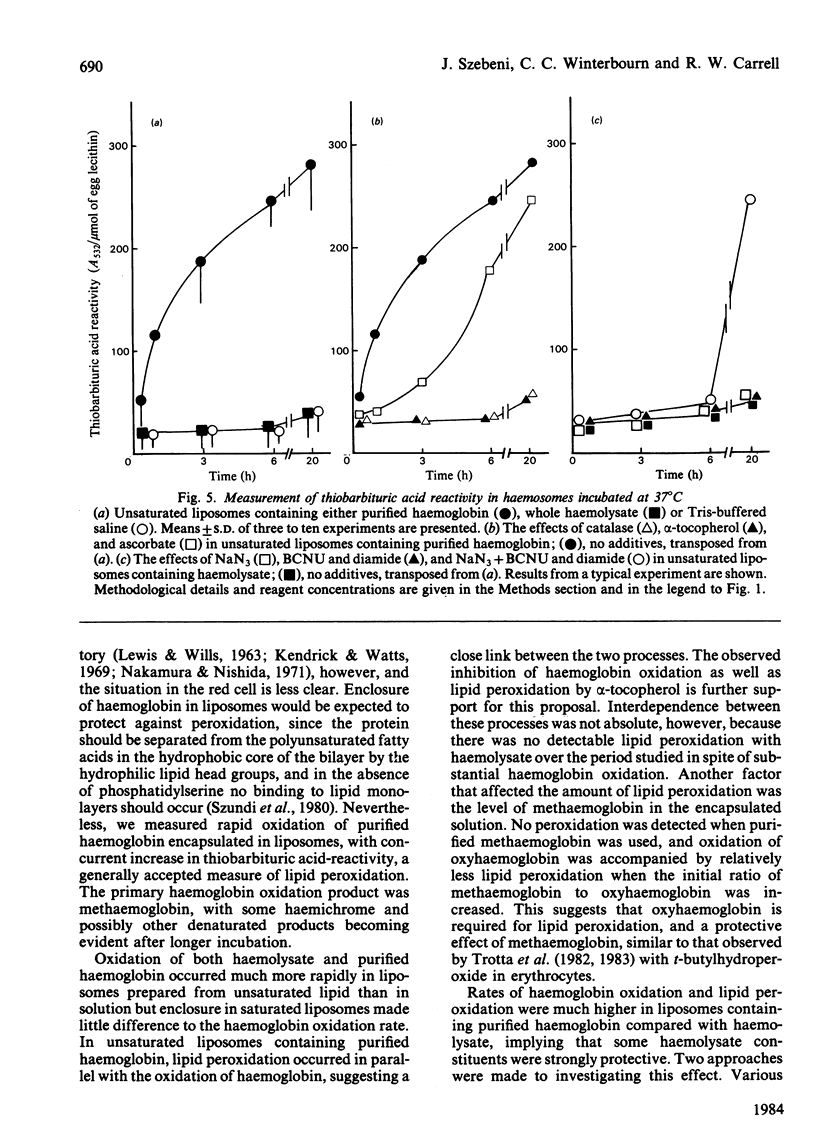
The relationship between haemoglobin and membrane oxidation was studied using liposomes containing haemoglobin (haemosomes) as a red cell model. Rapid oxidation occurred in haemosomes formed from purified haemoglobin and unsaturated lipid (egg phosphatidylcholines). After 3 h at 37 degrees C most of the haemoglobin was oxidized, predominantly to methaemoglobin with some haemichrome formation. The oxidation of haemoglobin was paralleled by membrane lipid peroxidation as measured by thiobarbituric acid reactivity. These changes were largely abolished by using freshly prepared haemolysate instead of purified haemoglobin, or when haemosomes were prepared with saturated phosphatidylcholines. In haemosomes consisting of fresh haemolysate and saturated phosphatidylcholine, the rate of haemoglobin oxidation at 37 degrees C corresponded to that of non-encapsulated haemolysate, and after 4 months storage at 4 degrees C 45% of oxyhaemoglobin was oxidized. In haemosomes prepared from purified haemoglobin and egg lecithin, alpha-tocopherol, catalase and ascorbate each protected against both haemoglobin oxidation and lipid peroxidation. Superoxide dismutase or reduced glutathione had no effect. In unsaturated-lipid haemosomes containing haemolysate, the rate of haemoglobin oxidation increased when catalase was inhibited or reduced glutathione was depleted, but after long term incubation only concurrent catalase-inhibition and glutathione depletion could increase thiobarbituric acid reactivity. These results demonstrate a close interdependence between haemoglobin oxidation and lipid peroxidation, and show that constituents of haemolysate strongly protect against both processes. H2O2 appears to be an important mediator, with its removal by either catalase or the glutathione/glutathione peroxidase system protecting against both oxidative changes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Djordjevich L., Miller I. F. Synthetic erythrocytes from lipid encapsulated hemoglobin. Exp Hematol. 1980 May;8(5):584–592. [PubMed] [Google Scholar]
- Frischer H., Ahmad T. Severe generalized glutathione reductase deficiency after antitumor chemotherapy with BCNU" [1,3-bis(chloroethyl)-1-nitrosourea]. J Lab Clin Med. 1977 May;89(5):1080–1091. [PubMed] [Google Scholar]
- Gaber B. P., Yager P., Sheridan J. P., Chang E. L. Encapsulation of hemoglobin in phospholipid vesicles. FEBS Lett. 1983 Mar 21;153(2):285–288. doi: 10.1016/0014-5793(83)80625-5. [DOI] [PubMed] [Google Scholar]
- Haurowitz F., Groh M., Gansinger G. Mechanism and kinetics of the hemin-catalyzed oxidation of linoleate in the oil-water interface. J Biol Chem. 1973 Jun 10;248(11):3810–3818. [PubMed] [Google Scholar]
- Huisman T. H., Dozy A. M. Studies on the heterogeneity of hemoglobin. IX. The use of Tris(hydroxymethyl)aminomethanehcl buffers in the anion-exchange chromatography of hemoglobins. J Chromatogr. 1965 Jul;19(1):160–169. doi: 10.1016/s0021-9673(01)99434-8. [DOI] [PubMed] [Google Scholar]
- Kendrick J., Watts B. M. Acceleration and inhibition of lipid oxidation by heme compounds. Lipids. 1969 Nov;4(6):454–458. doi: 10.1007/BF02531023. [DOI] [PubMed] [Google Scholar]
- Khachatur'ian A. A., Viazova E. P., Morozova G. M., Rozenberg G. Ia. Primenenie organicheskikh rastvoritelei dlia polucheniia ochishchennykh kontsentrirovannykh rastvorov gemoglobina. Probl Gematol Pereliv Krovi. 1979 Jan;24(1):58–60. [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M., Wertheim B., Correa W. S. Diamide, a new reagent for the intracellular oxidation of glutathione to the disulfide. Biochem Biophys Res Commun. 1969 Nov 6;37(4):593–596. doi: 10.1016/0006-291x(69)90850-x. [DOI] [PubMed] [Google Scholar]
- LEWIS S. E., WILLS E. D. Inhibition of the autoxidation of unsaturated fatty acids by haematin proteins. Biochim Biophys Acta. 1963 Jun 18;70:336–338. doi: 10.1016/0006-3002(63)90757-1. [DOI] [PubMed] [Google Scholar]
- Nicholis P. Contributions of catalase and glutathione peroxidase to red cell peroxide removal. Biochim Biophys Acta. 1972 Sep 15;279(2):306–309. [PubMed] [Google Scholar]
- Riggs A. Preparation of blood hemoglobins of vertebrates. Methods Enzymol. 1981;76:5–29. doi: 10.1016/0076-6879(81)76111-1. [DOI] [PubMed] [Google Scholar]
- Stocks J., Dormandy T. L. The autoxidation of human red cell lipids induced by hydrogen peroxide. Br J Haematol. 1971 Jan;20(1):95–111. doi: 10.1111/j.1365-2141.1971.tb00790.x. [DOI] [PubMed] [Google Scholar]
- Szebeni J., Breuer J. H., Szelenyi J. G., Bathori G., Lelkes G., Hollan S. R. Oxidation and denaturation of hemoglobin encapsulated in liposomes. Biochim Biophys Acta. 1984 Mar 22;798(1):60–67. doi: 10.1016/0304-4165(84)90010-2. [DOI] [PubMed] [Google Scholar]
- Szundi I., Szelényi J. G., Breuer J. H., Bérczi A. Interactions of haemoglobin with erythrocyte membrane phospholipids in monomolecular lipid layers. Biochim Biophys Acta. 1980;595(1):41–46. doi: 10.1016/0005-2736(80)90245-x. [DOI] [PubMed] [Google Scholar]
- TAPPEL A. L. Unsaturated lipide oxidation catalyzed by hematin compounds. J Biol Chem. 1955 Dec;217(2):721–733. [PubMed] [Google Scholar]
- Thornalley P. J., Trotta R. J., Stern A. Free radical involvement in the oxidative phenomena induced by tert-butyl hydroperoxide in erythrocytes. Biochim Biophys Acta. 1983 Aug 23;759(1-2):16–22. doi: 10.1016/0304-4165(83)90183-6. [DOI] [PubMed] [Google Scholar]
- Trotta R. J., Sullivan S. G., Stern A. Lipid peroxidation and haemoglobin degradation in red blood cells exposed to t-butyl hydroperoxide. Effects of the hexose monophosphate shunt as mediated by glutathione and ascorbate. Biochem J. 1982 May 15;204(2):405–415. doi: 10.1042/bj2040405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta R. J., Sullivan S. G., Stern A. Lipid peroxidation and haemoglobin degradation in red blood cells exposed to t-butyl hydroperoxide. The relative roles of haem- and glutathione-dependent decomposition of t-butyl hydroperoxide and membrane lipid hydroperoxides in lipid peroxidation and haemolysis. Biochem J. 1983 Jun 15;212(3):759–772. doi: 10.1042/bj2120759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursini F., Maiorino M., Ferri L., Valente M., Gregolin C. Hydrogen peroxide and hematin in microsomal lipid peroxidation. J Inorg Biochem. 1981 Oct;15(2):163–169. doi: 10.1016/s0162-0134(00)80300-1. [DOI] [PubMed] [Google Scholar]
- Wendel A. Glutathione peroxidase. Methods Enzymol. 1981;77:325–333. doi: 10.1016/s0076-6879(81)77046-0. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., McGrath B. M., Carrell R. W. Reactions involving superoxide and normal and unstable haemoglobins. Biochem J. 1976 Jun 1;155(3):493–502. doi: 10.1042/bj1550493. [DOI] [PMC free article] [PubMed] [Google Scholar]


