Abstract
Alcohol-associated liver disease (ALD) is a significant global health challenge, encompassing a spectrum from steatotic liver disease to cirrhosis and alcohol-associated hepatitis, and contributed to 25% of global cirrhosis deaths in 2019. The identification of both modifiable (e.g. heavy drinking, metabolic syndromes) and non-modifiable risk factors (e.g. genetic predispositions) is crucial for effective disease management. Alcohol use assessment and treatment, by using both behavioral therapy and pharmacotherapeutic modalities, nutrition support, and optimization of liver disease modifiers, form the cornerstone of management. Advances in medical therapies, such as fecal microbiota transplantation and novel agents such as IL-22, are being explored for their therapeutic potential. A unifying theme in ALD care is the need for a personalized approach to management, accounting for the spectrum of the disease and individual patient characteristics, to tailor interventions effectively. Finally, it is essential to address the challenges to effective ALD treatment, including socioeconomic, logistical, and stigma-related barriers, to improve patient outcomes. This review discusses the current knowledge on ALD, including epidemiology, pathophysiology, risk factors, and management strategies, highlighting the critical role of integrated care models.
Keywords: alcohol-associated liver disease, alcohol use disorder, alcohol-associated hepatitis, alcohol-associated cirrhosis, steatotic liver disease
Introduction
Liver disease that is related to heavy alcohol consumption is a significant global health issue [1]. Deaths linked to alcohol-associated liver disease (ALD) accounted for 25% of global cirrhosis deaths in 2019 [2] and are predicted to increase [3]. Liver damage caused by alcohol use begins with hepatic steatosis that progresses histologically to steatohepatitis and fibrosis, eventually resulting in more advanced disease including cirrhosis and alcohol-associated hepatitis (AH) [4]. Of note, AH occurs in a minority of patients with alcohol use disorder and is not a necessary occurrence on the path from steatosis to cirrhosis. As such, ALD is part of the spectrum of steatotic liver disease (SLD) [5]. The definition of a “ standard drink” and, in turn, the definition of risky drinking vary by country [6]. The National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the Centers for Disease Control and Prevention (CDC) in the USA define heavy drinking as ≥4 drinks (56 g) on any given day or ≥8 drinks (112 g) per week for women and ≥5 drinks (70 g) on any given day or ≥15 drinks (210 g) per week for men over an extended period [7]. Alcohol use disorder (AUD), characterized by excessive and problematic drinking patterns, is defined as having ≥2 of 11 of the Fifth Edition of Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria within the preceding 12 months [8]. Moderate to severe AUD (at least four criteria) is observed in ≤80% of patients with ALD [9]. While >40% of the global population reports alcohol use [10], in the USA, 13.6% of men (≥18 years) and 8.9% of women (≥18 years) have an AUD [11, 12]. The number of women with AUD is increasing more rapidly in the USA and women often have more severe effects of AUD than men [13]. Alcohol-associated steatohepatitis develops in 10%–35% of patients with heavy alcohol drinking with hepatic steatosis [14]. Progression beyond steatosis is variable among patients and not all patients with AUD progress to severe disease. However, 8%–20% of patients with a history of heavy alcohol use with alcohol-related steatohepatitis will develop alcohol-associated cirrhosis (AC) [15]. The prevalence of ALD in the US population in 2022 was 5.0% (95% confidence interval [CI], 2.9%–7.6%) [16]. ALD is one of the primary causes of liver cirrhosis and liver transplantation globally, and the main cause of cirrhosis and cirrhosis death in the USA [17–20].
Metabolic dysfunction and alcohol-associated liver disease (MetALD), which is a new category, has been defined to include patients with both cardiometabolic risk factors for metabolic dysfunction-associated steatotic liver disease (MASLD) and alcohol intake exceeding the new upper limit for MASLD (previously known as non-alcoholic fatty liver disease) [5]. The level of alcohol consumption that is used to define MetALD is approximately the same as the level that NIAAA/CDC use to define heavy drinking [7]. This new classification not only recognizes the combined effects of alcohol and metabolic factors on liver disease, but also offers a more nuanced framework for understanding and categorizing these conditions as part of a spectrum [21].
Considering the high prevalence and varied impact of ALD, which spans a range of presentations and severities, a variety of management strategies are available for ALD, AH, and AC. A unifying theme in the treatment of all patients with AUD and ALD is the need for holistic and integrated management that encompasses shared ownership of common objectives, mutual reliance among providers, adaptable role assignments, and the introduction of new professional tasks [22]. Our review seeks to emphasize the shared strategies in managing patients with ALD while also delineating the distinct approaches that are tailored to each specific condition.
ALD risk stratification
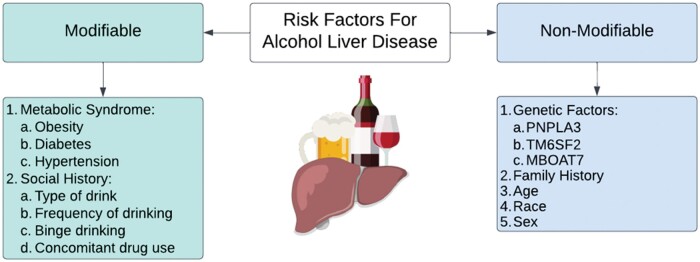
The most important determinant of outcomes for ALD is the stage of fibrosis [23]. Therefore, an understanding of the risk factors for the development of fibrosis and progression to more advanced stages is vital. Several non-modifiable risk factors increase the progression of ALD. The risk of progressing to ALD is notably higher in patients with a mutation in the lipid droplet protein, patatin-like phospholipase domain-containing 3 (PNPLA3) and is enriched in Hispanic individuals. This polymorphism, which is also closely linked to MASLD, significantly increases the likelihood of developing ALD [24–28]. Another study that used a US population-based cohort demonstrated the modifying effect that the alcohol exposure level has on liver disease severity [29]. In Hispanic individuals, it is not currently known how much of an increased risk of ALD is attributable to PNPLA3 vs other known and unknown factors [30]. However, compared with European Americans (23%) and African Americans (17%), individuals of Hispanic descent had a higher frequency (49%) of PNPLA3 [31]. One study examined this question in SLD, but not specifically ALD, and found that the genetic differences among Hispanic individuals contributed to, but did not fully explain, the differences in SLD prevalence [32]. Moreover, the progression of ALD to more severe forms such as AC has been associated with novel genetic loci such as transmembrane 6 superfamily member 2 (TM6SF2), membrane-bound O-acyltransferase 7 (MBOAT7), and 17 beta hydroxysteroid dehydrogenase 13 (HSD17B13) [33, 34]. Loss of function of the rs72613567 variant in HSD17B13 results in protection against ALD [35]. Moreover, Chinese Han males were found to have a higher rate of specific single nucleotide polymorphisms of HSD17B13, which results in increased protection against ALD [36]. In addition, those with female sex, older age, and Hispanic descent seem to have an increased risk of ALD [13, 19, 37, 38]. Modifiable risk factors also play a role in the development and progression of ALD. Metabolic co-morbidities such as obesity and diabetes have been shown to increase the risk of ALD among heavy drinkers [39–41]. Among genetically predisposed individuals, the risk of developing cirrhosis due to heavy alcohol consumption was significantly increased in patients with diabetes, with an odds ratio (OR) reaching ≤17.1 (95% CI, 11.3–25.7) in a cohort study that pooled genomic data from three international biobanks [42]. Obesity has been shown to increase the risk of mortality among patients who consume 1–14 units of alcohol per week by a relative risk (RR) of 5.44 (95% CI, 1.40–21.1) [43]. A family history of either ALD or metabolic conditions also increases the likelihood of developing ALD [44, 45]. Finally, the drinking frequency, type of drinks chosen (e.g. there is an association between exclusive liquor/cocktail consumption and at-risk liver fibrosis in patients with SLD but more data are needed to fully understand this association), binge drinking, and concomitant drug use have been shown to play a role in the risk of ALD development among AUD patients (Figure 1) [29, 46–49].
Figure 1.
Risk factors that are associated with the progression of alcohol use disorder into alcohol-associated liver disease. PNPLA3 = patatin-like phospholipase domain-containing protein 3, TM6SF2 = transmembrane 6 superfamily member 2, MBOAT7 = membrane-bound O-acyltransferase domain-containing 7.
The FIB-4 score can predict the extent of fibrosis in patients with liver disease, including those with ALD [50–53]. The enhanced liver fibrosis (ELF) test and FibroTest can be used to effectively exclude advanced liver fibrosis with cut-off values of <10.5 and <0.58, respectively. For the detection of advanced stages of fibrosis (F3–F4), an FIB4 score of ≥3.25 has a positive predictive value (PPV) of 64% (95% CI, 51%–76%) and a negative predictive value (NPV) of 88% (95% CI, 83%–92%), while an ELF test value of ≥10.5 has a PPV of 71% (95% CI, 59%–81%) and an NPV of 94% (95% CI, 89%–96%) among patients with ALD [54]. Moreover, among heavy-drinking patients with advanced liver disease, a FibroTest value of ≥0.58 had a PPV of 60% (95% CI, 48%–72%) and an NPV of 90% (95% CI, 85%–94%) [54]. The use of transient elastography (TE) is an effective modality for assessing liver stiffness and fibrosis among patients with ALD. One study demonstrated that a TE cut-off reading of ≥19.7 kPa yielded a PPV of 88% (95% CI, 76%–95%) and an NPV of 96% (95% CI, 92%–98%) for advanced fibrosis (≥F3) [54]. Despite the use of non-invasive testing, these tests are helpful in slowly progressive diseases such as hepatitis C virus and MASLD, although they remain unreliable during periods of active drinking [55].
AH is a severe form of ALD with a clinical history of jaundice onset within 8 weeks in the setting of significant alcohol use and a characteristic biochemical pattern of an aspartate aminotransferase/alanine aminotransferase (AST/ALT) ratio of ≥1.5 with AST of ≥50 IU/L, both AST and ALT of <400 IU/L, and total bilirubin of >3 mg/dL as defined by a consortium of investigators and endorsed by the American Association for the Study of Liver Diseases [56]. The definition of AH by the NIAAA, while very useful, does not distinguish between the severe inflammatory/steatotic forms of AH and decompensated cirrhosis. Future studies should aim to identify tools that help to distinguish the differences in AH. Several prognostic models have been developed to address AH severity. The Maddrey discriminant function (MDF) [57], consisting of a combination of prothrombin time and total bilirubin, was the first model to assess AH prognosis and the potential benefit of corticosteroid treatment [58, 59]. However, there are some concerns that the MDF is limited in its ability to capture patients with less severe disease who may have otherwise benefited from early identification and treatment [60]. Despite the limited predictive value of the MDF score, its merit lies in its ability to focus on liver injury instead of renal injury in clinical trials [61]. The Model for End-stage Liver Disease (MELD) score, calculated by using renal function, bilirubin, and international normalized ratio (INR), has been used extensively to assess the severity of AH [56]. Other iterations of the MELD score such as the MELD-Na score have not been as well validated in patients with AH. In fact, in AH patients without ascites, the MELD-Na score should be used with caution, as it may overestimate the mortality risk [62]. The Age, Bilirubin, INR, Creatinine (ABIC) score, integrating age, broadens assessment but cannot be used to decide steroid use and cannot accurately predict mortality in severely ill patients [63, 64]. The Glasgow Alcoholic Hepatitis Score (GAHS), while offering improved mortality specificity, is not as commonly utilized [65, 66]. A multicenter study compared the prognostic ability of these models to predict 28- and 90-day mortality across different countries [67]. The area under the curve (AUC) values for MELD and MELD-Na showed moderate and very good predictive accuracy, respectively. For example, MELD-Na in Spain reached an AUC of ≤0.875. In contrast, MDF generally offered lower AUC values, with less reliability in some countries such as Mexico, where the AUC was only 0.677–0.686. The GAHS and the ABIC score also show a wide range of predictive accuracies, with the GAHS reaching ≤0.897 in Korea and the ABIC achieving 0.880 in Spain, showcasing their potential in specific scenarios. However, the varying scores that were developed in different population groups may not necessarily result in the same outcomes in varying populations and locations. A six-plasma protein panel was identified that included three poor-prognosis-associated proteins (interleukin-1 receptor-like 1, lymphocyte cytosolic protein 2, and antileukoproteinase) and three good-prognosis-associated proteins (collagen α-1[I] chain, protein S, and thrombospondin-2) [68]. This panel was derived as a surrogate for a 123-gene hepatic transcriptomic signature to predict the prognosis of patients with severe alcoholic hepatitis [68]. The six-plasma-protein panel surrogate for a 123-gene signature was combined with MELD to create the ps-MELD score [68]. In the validation cohort, the high-risk ps-MELD group (40% of the cohort) was significantly associated with death or liver transplantation within 90 days (adjusted hazard ratio [aHR], 4.57; 95% CI, 2.15–9.30; P < 0.001) [68]. The ps-MELD score offers a non-invasive method for early prognosis and treatment decisions in severe AH [68]. Biomarkers such as CK-18 and CK-19 have limited use in assessing prognosis in AH, and potentially the response to corticosteroids also [69, 70]. A recent study from the AlcHepNet consortium demonstrated that IL-13 and age predict 90-day mortality in severe AH [71]. Of note, the fibrosis scores that were discussed in the preceding paragraph have not been validated (and may be misleading) in patients with AH. In conclusion, the discussed scores exhibit several overlapping variables, which contribute to their interdependence and hinder their effectiveness as unique predictive tools. To address this overlap and refine predictions for patients with ALD, the incorporation of independent factors such as liver size could be beneficial. By doing so, we can potentially improve the accuracy and reliability of these predictions.
ALD management
AUD management
Although the stage of fibrosis is an important determinant of survival outcomes in ALD, abstinence from alcohol is associated with a marked improvement in survival rates for both compensated and decompensated ALD [23]. Abstinence remains the fundamental clinical tenet in the management of ALD to enhance patient prognosis and survival, and therefore merits discussion first [23, 72, 73]. Among patients with AC, a significant reduction in the risk of hepatic decompensation (aHR, 0.39; 95% CI, 0.28–0.56), as well as liver-related (aHR, 0.43; 95% CI, 0.26–0.70) and all-cause (aHR, 0.45; 95% CI, 0.30–0.69) mortality was evident in those patients who remained abstinent [74]. Moreover, abstinence was shown to increase survival after 1.5 years (HR, 0.51; 95%CI, 0.33–0.81), with this significantly improved survival being present up until 5 years post-abstinence among patients with AC [75]. Therefore, the evaluation and management of AUD are a critical part of the management of patients with ALD [76]. Studies have shown that employment, higher educational status, and presence of a spouse may be important factors in fostering abstinence, while life stressors such as the death of a loved one can be triggers for individuals to return to drinking [77, 78]. While abstinence is the goal, recent studies have also highlighted that a reduction in alcohol consumption, without complete abstinence, can result in improved long-term outcomes [76, 79].
Behavioral, psychosocial, and medical therapies are all important ways to promote abstinence in patients with AUD. Twelve-step facilitation programs, such as Alcoholics Anonymous, have been shown to be significant in enabling long-term abstinence among AUD patients[80]. Psychosocial treatments, including cognitive behavioral therapy, motivational interviewing, and brief intervention therapies [81], significantly impact drinking outcomes; a study of 482 alcohol-dependent adults who received primarily psychosocial treatment showed a 30-day abstinence rate of 57% in the treatment group vs 12% in a comparison group from the general population (OR, 14.67; 95% CI, 6.45–33.38). Additionally, 40% of the treated group vs 23% of the untreated group reported no binge drinking, psychosocial problems, or alcohol dependence symptoms (OR, 7.30; 95% CI, 3.49–15.30) [82]. Motivational interviewing and therapy, using open-ended non-judgmental questions, and mobile health treatments have been shown to be useful in decreasing drinking and promoting abstinence among patients with AUD [83–90].
There are several pharmacological options that can be used to reduce cravings and promote abstinence among patients with AUD. First-line medical treatment includes naltrexone, which is a mu-opioid receptor blocker that works by curbing the reward feeling of alcohol [91], and acamprosate, which modulates glutamate neurotransmission at the metabotropic-5 glutamate receptor and results in reduced alcohol cravings [92]. Disulfiram, which inhibits the metabolism of acetaldehyde [93], should not be used in patients with ALD due to potential hepatotoxicity [94]. Baclofen has been studied in advanced liver disease with varying degrees of success [95]. The dosing, efficacy, and nuances of these pharmacological options, including topiramate [96, 97] and gabapentin [98], are outlined in Table 1.
Table 1.
Medical management options for abstinence among patients with AUD
| Treatment | Dosing | Mechanism of action | FDA-approved for abstinence | Evidence | Adverse effects | Cautions/contraindications |
|---|---|---|---|---|---|---|
| First-line treatment | ||||||
| Naltrexone | Oral: 50 mg/day, long-acting injectable (LAI): 380 mg IM every 4 weeks | Mu-opioid receptor blockade, modifies hypothalamic–pituitary–adrenal axis | Yes |
|
Nausea, fatigue, decreased appetite, injection-site reactions for long-acting injectables | Use of opioids, acute hepatitis, hepatic failure |
| Acamprosate | 666 mg three times daily, adjusted for renal function and body weight | Modulation of glutamate neurotransmission at metabotropic-5 glutamate receptors | Yes | NNT 12 | Diarrhea, nervousness, fatigue | Severe renal dysfunction (creatinine clearance ≤30 mL/min) |
| Baclofen | 5 mg TID and uptitrate q 3–5 days (max. 45 mg daily) | GABAB receptor agonist | No | 68%–71% vs 24%–29% placebo for abstinence | Hepatotoxicity, sleep apnea, muscle spasms | Renal dysfunction, mental/mood disorders (such as schizophrenia), brain disorders (such as seizures, stroke) |
| Second-line treatment | ||||||
| Disulfiram | 250–500 mg/day initially, maintenance 250 mg/day | Accumulation of acetaldehyde, causing unpleasant effects | Yes | Not efficacious | Fatigue, mild drowsiness, headache, dermatitis, severe reactions rare | Clinically significant coronary artery disease, psychosis, known hypersensitivity, possible hepatotoxicity; should be avoided in patients with ALD |
| Topiramate | Start at 25 mg/day, up to 300 mg/day over 8 weeks | Blocks voltage-dependent sodium channels, potentiates GABA transmission, antagonizes glutamate receptors | No | rs2832407*C-homozygotes NNT: 2.28 | Cognitive impairment, paresthesia, weight loss, headache, fatigue, dizziness, depression | None specified, caution in patients with a history of kidney stones or glaucoma |
| Gabapentin | Varies; typically, 900–3,600 mg/day in divided doses | Not fully understood; believed to affect calcium channels and reduce neurotransmitter release | No |
|
Dizziness, fatigue, ataxia, edema, weight gain, potential for abuse | Use with caution in patients with renal impairment; avoid abrupt discontinuation |
AUD = alcohol use disorder, FDA = Food and Drug Administration, LAI = long-acting injectable, mg = milligram, IM = intramuscular, NNT = number needed to treat, TID = ter in die, ALD = alcohol-associated liver disease.
The COMBINE Study by Anton et al. [99] found that a combination of naltrexone with medical management and/or behavioral therapy (Combined Behavioral Intervention) was effective in increasing alcohol abstinence and reducing heavy drinking among patients with alcohol dependence. Naltrexone alone or combined with behavioral intervention provided significant benefits, while acamprosate showed no added efficacy. However, the benefits that were observed during treatment tended to diminish after 1 year without continued intervention. Overall, naltrexone with medical management was identified as a key treatment strategy for alcohol dependence [99].
Multidisciplinary approach to early ALD management
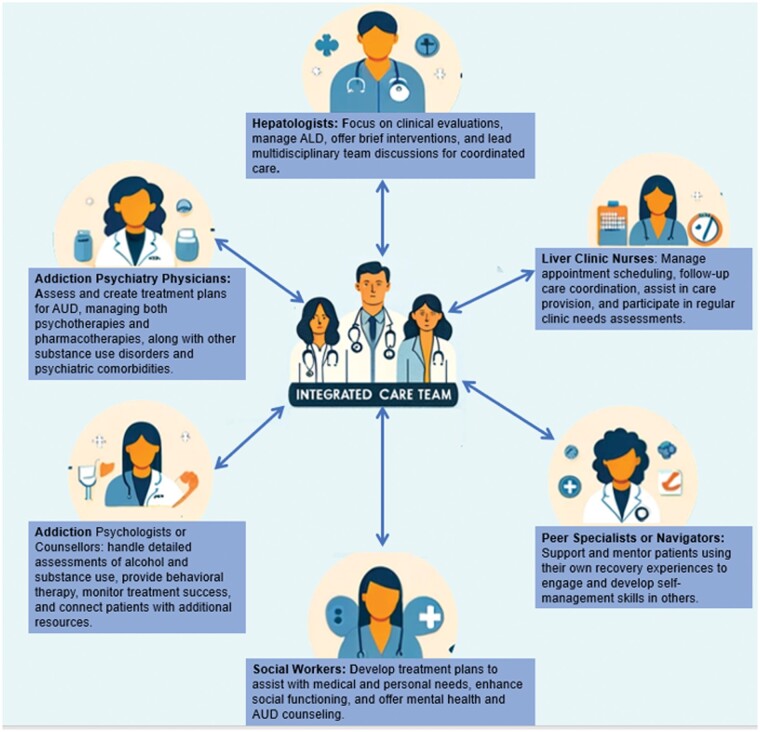
Alcohol cessation leads to improvements particularly in hepatic steatosis within 2 weeks [75, 100, 101]. On the other hand, continued drinking has clearly been demonstrated to increase the risk of progression of ALD and fibrosis, including increasing portal pressure and its complications such as variceal bleeding [100–102]. Therefore, optimally, patients with ALD are identified at an early stage to enable impactful interventions, particularly the targeting of AUD, to mitigate disease progression risk. A recent development in AUD care among those with ALD has been the development of integrated care models (ICM) by using a multidisciplinary team to manage both AUD and ALD concomitantly. Hepatologists provide clinical evaluation and management of ALD, which include conducting brief interventions. Addiction psychiatrists tailor treatment plans that encompass both psychotherapies and pharmacotherapies for AUD, while addressing any comorbid psychiatric conditions. Addiction psychologists or counselors provide behavioral therapy, assess alcohol and substance use history, and ensure treatment efficacy by adjusting therapy frequency and connecting patients with resources. Liver clinic nurses play a crucial role in care coordination, from scheduling appointments to supporting the medical team. Social workers focus on enhancing patients’ social functioning by addressing personal needs through tailored treatment plans and interventions. Additionally, peer specialists or navigators leverage their personal recovery experiences to offer unique support and mentorship, helping patients to develop essential self-management skills. Together, this team fosters a holistic and integrated approach to the treatment of AUD and ALD [103]. These models have been shown to reduce alcohol relapse yet are difficult to implement across healthcare centers (Figure 2) [104–108].
Figure 2.
Integrated care model for the management of AUD. AUD = alcohol use disorder, ALD = alcohol-associated liver disease.
The successful implementation of multidisciplinary ALD treatment models faces several potential barriers, including financial sustainability and patient attrition. Logistical complexities require clinics to navigate diverse diagnostic codes, secure separate insurance authorizations, and accommodate infrastructural needs for both medical and psychiatric services. Geographic and insurance coverage disparities further complicate access, due to the significant travel and expenses that are incurred from the separation of psychiatric and substance use disorder care from liver specialty services. Additionally, impaired patient cognitive status may pose a challenge, as hepatic encephalopathy (HE) in decompensated ALD patients may be mistakenly viewed by mental health providers as a barrier to treatment, despite the potential for both ALD and HE treatment. These barriers underscore the challenges to ensuring the sustainability and effectiveness of ALD treatment models across financial, administrative, geographic, and clinical domains [103, 109]. Moreover, other barriers to ALD treatment include provider- and patient-related challenges such as lack of training and familiarity with AUD treatment among providers and a significant impact of societal stigma. This stigma exacerbates patient reluctance to seek help, further hindered by socioeconomic and cultural disparities that limit access to and participation in treatment programs. Several potential solutions to improving the efficacy of AUD treatments have been proposed, including integrated AUD management, broadening insurance coverage, and addressing socioeconomic and racial disparities that are prevalent in AUD detection and management. A summary of these barriers and solutions can be found in Table 2 [110].
Table 2.
Barriers associated with treatment of AUD
| Barrier | Examples/description | Possible solutions |
|---|---|---|
| Provider and training deficiencies | Lack of training and familiarity with AUD treatment among providers |
|
| Patient access and socioeconomic factors | Financial barriers, insurance limitations, and lack of transportation hinder access to treatment |
|
| Disproportionate impacts on historically underrepresented racial/ethnic and socioeconomic groups, leading to inequities in treatment access and outcomes |
|
|
| Stigma and cultural barriers | Societal stigma and discrimination associated with AUD deter patients from seeking treatment |
|
| Cultural and linguistic mismatches between healthcare providers and patients can exacerbate barriers to accessing and engaging with treatment |
|
|
| Lack of personnel | Limited research funding and hospital staff dedicated for research recruitment and clinical trials |
|
AUD = alcohol use disorder.
In addition to abstinence, nutritional optimization is a critical component in the management strategy of all stages of ALD. Daily nutrition should target 1.2–1.5 grams of protein per kilogram per day, along with total calorie consumption of 35 kcal per kilogram per day [111, 112]. Moreover, the randomized–controlled trial that was conducted by Plank et al. [113] clearly indicated that nighttime feeding resulted in significantly improved nutritional status among patients with cirrhosis. In patients with ALD, myokines such as myostatin and decorin play significant roles in disease progression and nutritional impact. Elevated myostatin levels, which negatively regulate muscle mass, are associated with increased fibrosis, systemic inflammation, and organ failures, particularly in severe stages such as acute-on-chronic liver failure (ACLF) [114]. Conversely, decorin, which promotes muscle hypertrophy and counters myostatin, is reduced in more severe forms of ALD. This imbalance contributes to malnutrition, sarcopenia, and poor outcomes, including higher mortality rates [114]. The study by Kaur et al. [114] showed that patients with high myostatin and low decorin levels exhibited worse nutritional status, higher disease severity, and a nearly 19-fold increased risk of 30-day mortality. Decorin, when combined with MELD scores, improved the predictive accuracy for mortality and disease progression in ALD, highlighting its potential as a therapeutic target [114]. Proper nutritional intervention, compared with no nutritional management, reduces mortality risk in patients with either AC or AH (RR, 0.80; 95% CI, 0.64–0.99) [115].
AH management
Given the importance of severe AH as a cause of ALD-related mortality, the Alcoholic Hepatitis Network (AlcHepNet) was established. The AlcHepNet is a collaboration among US medical institutions that aims to coordinate investigation and consolidate patient data and samples to enhance the understanding and treatment of AH. By creating a centralized database and bio-specimen bank, AlcHepNet seeks to advance research on epidemiology, diagnosis, pathophysiology, natural history, and treatment options of AH, aiming for improved patient care for this serious liver disease [116].
Corticosteroids
A wide array of pharmacological treatments has been developed for AH (Table 3); however, corticosteroids remain the widely endorsed first-line treatment, as alternatives have failed to demonstrate acceptable efficacy and safety at the current time. The mechanism of action for prednisolone in AH treatment is the reduction of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and an increase in anti-inflammatory cytokines such as interleukin 10 [117]. The seminal US multicenter study by Carithers et al. [57] showed that 32 mg of methyl-prednisolone daily improved survival at 28 days in patients with AH and MDF of ≥32. Another study, by Mathurin et al. [118], showed that the use of prednisolone in AH patients resulted in significantly improved 1-year survival in the treated cohort (69%; 95% CI, 57%–81%) compared with a nontreated cohort (Group II, 41%; 95% CI, 23%–59%; P = 0.01). In 2008, a Cochrane meta-analysis noted that corticosteroids significantly lowered mortality among patients with a MDF score of >32 or those with spontaneous HE [119]. Subsequently, the STOPAH study, which was a randomized, double-blind, controlled trial of prednisolone and/or pentoxifylline in patients with severe AH, demonstrated a reduced adjusted odds of mortality with prednisolone use compared with placebo (OR, 0.61; 95% CI, 0.41–0.91, P = 0.02) at 30 days, but not at 90 days [120]. A comprehensive meta-analysis confirmed the short-term mortality benefit of corticosteroid use in severe AH with an HR of 0.64 (95% CI, 0.48–0.86) [121]. The Lille score was developed to predict survival at 6 months following treatment of severe AH with corticosteroids [122, 123]. It has also been used to determine which individuals are unlikely to benefit from ongoing corticosteroids after 7 days of treatment and should therefore be considered for alternative management. Of note, the Lille score at 4 days of steroid treatment has been shown to be similar in outcome prediction to the traditional 7-day Lille score, and can be considered when assessing early steroid response [124]. A Lille score of >0.45 after 1 week has been established as a cut-off point for the discontinuation of steroid therapy, as these patients no longer benefit from steroids [125]. Moreover, on its own, the Lille score is not as accurate at predicting 28-day survival; instead, a combination of MELD and Lille was the best at predicting 28-day mortality, with an accuracy of 0.90 [126]. Furthermore, the use of steroids increases the risk of specific infections and thus, in the presence of infection complications, steroids should be halted to reduce the risk of further sequalae [127].
Table 3.
Potential medical therapies for AH
| Treatment | Mechanism of action | Clinical trial outcomes | Side effects | Additional considerations |
|---|---|---|---|---|
| Corticosteroids (prednisolone, prednisone, methyl-prednisolone) | Anti-inflammatory | Reduction in short- to medium-term mortality, but increased risk of infections and no long-term mortality benefit | Weight gain, indigestion, sepsis, gastro-intestinal hemorrhage, restlessness | Controversial due to risks of sepsis and hemorrhage; effectiveness limited to short-term |
| Pentoxifylline | Hemorrhagic agent | Initially showed short-term benefits; recent studies show no benefit | Gastro-intestinal complaints, dizziness, headaches, flushing | Controversial due to initial positive results; however, recent results showed no benefit |
| Bovine colostrum (IMM-124E) | IgG to LPS, reduces bacterial translocation | Decrease in serum endotoxin levels at 7 months | Gastro-intestinal complaints, rash, unpleasant taste | Phase II, not recruiting |
| Lactobacillus rhamnosus GG | Changes in gut microbiome | Decreased liver injury 1 month post-LGG therapy | Rash, pruritis, swelling, dizziness, throat swelling | RCT |
| Amoxicillin-clavulanate | Interference with bacterial cell wall synthesis | No difference to prednisolone alone | Gastro-intestinal complaints, rash | RCT |
| Anakinra (+ zinc) | IL-1 receptor antagonist; anti-inflammatory; immunomodulation | Survival at 6 months; improved outcomes in combination but not statistically significant mortality benefit | Infection risks given immunomodulatory effect | Combined with zinc and pentoxifylline in trials; requires further investigation |
| Selonsertib (GS-4997) | ASK-1 antagonist, inhibits MAPK, JNK, p38 | Safety and serious adverse events at 28 days plus 30 days | Headache, nausea | Phase II, completed |
| Emricasan (IDN-6556) | Pan-caspase inhibitor | Survival at 28 days | High-blood-level concerns, headache, nausea, fatigue | Study terminated after five patients |
| Larsucosterol | Epigenetic modulator inhibiting DNA methylation | Reduces risk of mortality (41% reduction, P = 0.07) among AH patients | No unexpected serious adverse events | May now enter a Phase 3 trial with the goal of Food Drug Administration (FDA) approval in the near future |
| Obeticholic acid (INT-747) | FXR activation, bile acid agonist, anti-inflammatory | Change in MELD score at 6 weeks | Pruritus, fatigue, gastro-intestinal disorders | Phase II, completed |
| Metadoxine | Antioxidant; promotes abstinence; increases hepatic glutathione concentrations | Survival at 30 days; improved survival and sobriety rates in small trials | Nausea, upset stomach, diarrhea | Phase IV, recruiting; dual effects on liver health and sobriety maintenance promising but require larger studies |
| IL-22 (F-652) | Anti-inflammatory and hepatic regeneration | Safety and serious adverse events at 42 days; preliminary data showed efficacy signals in a small cohort study | Dermal inflammation, ancanthosis | Phase I completed; Phase IIb RCT underway |
| G-CSF (filgrastim) | Increases neutrophils, hepatic regeneration | Survival at 2 and 6 months depending on CS response; mortality benefit in small clinical studies | Bone pain, injection-site reactions | Phase IV, active and recruiting; results from larger trials are eagerly awaited |
| Infliximab | TNF-α inhibitor | Improvement in Maddrey's score; increased infection risk | Increased risk of infections | Not recommended due to serious infection risk |
| Etanercept | TNF-α inhibitor | Similar mortality rates to placebo at 1 month; increased 6-month mortality | Higher rate of serious infections | Not effective; poses a risk of serious infections |
| N-acetylcysteine (NAC) | Antioxidants | Some promise shown in combination with steroids | Dry mouth, nausea, vomiting | Encouraging data; further studies needed |
| Rifaximin | Antibiotic with low systemic absorption; targeting gut–liver axis; reduces endotoxin levels | Decreased endotoxin levels and hepatic venous pressure gradient; trend toward benefit in small pilot study | Generally well tolerated with low systemic absorption | Positive effects on liver hemodynamics; pilot study |
| Betaine | Antioxidant and methionine metabolite | Improvement in hepatic steatosis in case reports | Diarrhea, nausea | Needs further study |
| Granulocytapheresis | Removes activated granulocytes and monocytes | No benefit in case series of severe AH patients | Well tolerated without hemodynamic compromise | No clinical benefit observed |
AH = alcohol-associated hepatitis, IgG = immunoglobulin G, LPS = lipopolysaccharide, IL1 = interleukin 1, ASK-1 = apoptosis signal regulating kinase 1, MAPK = mitogen activated protein kinase, JNK = jun N terminal kinase, FXR = farnesoid receptor X, IL-22 = interleukin 22, TNF-α = tumor necrosis factor alpha, G-CSF = granulocyte stimulating factor.
Anti-TNF therapy
An elevated serum level of TNF-α was found to be a predictor of mortality in severe AH [128]. Pentoxifylline (PTX), which is a TNF-α inhibitor, was shown to reduce the incidence of hepatorenal syndrome and mortality in severe AH patients in 1991 [129]. While a follow-up trial by Akriviadis et al. [130] in 2000 also suggested that PTX had a short-term survival benefit for severe AH, the STOPAH trial did not show any mortality benefit for PTX and, thus, PTX is now largely out of favor as a treatment modality [120]. Infliximab, which is an anti-TNF antibody, combined with prednisolone significantly increased the risk of serious infections without improving survival rates in patients with severe AH [131]. Consequently, the trial was prematurely halted, emphasizing the complexity of treating AH and the need for cautious clinical evaluation of TNF-α antagonists in this context [131, 132]. Etanercept, which is the soluble TNF-α receptor, also significantly increased 6-month mortality for AH [133].
Antibiotics
Infections are a major cause of morbidity and mortality in patients with severe AH [134]. Rifaximin, which is a non-absorbable broad-spectrum antibiotic that is used with corticosteroids in severe AH, showed promise for the reduction of bacterial infections and liver-related complications over 90 days compared with a historical control group. This multicenter pilot study also observed a trend towards reduced mortality and ACLF rates in the rifaximin group, indicating its potential safety and efficacy in managing severe AH [135]. Amoxicillin-clavulanate combined with prednisolone reduced infection rates, but there were no increased survival benefits compared with prednisolone alone [136]. Anakinra, which is an interleukin 1 receptor blocker, was shown to reduce liver injury in a rodent model of alcohol-related liver injury [137].
Anti-IL-1 therapy
The DASH trial evaluated the efficacy of a combination treatment (COMB) by using anakinra for 14 days, pentoxifylline for 28 days, and zinc for 180 days compared with 4 weeks of treatment with 32 mg of methyl-prednisolone in patients with severe AH. At 180 days, the survival rates were 67.9% for COMB and 56% for corticosteroids, although they were not statistically different (HR, 0.69; P = 0.30). Although the overall rates of infections were similar, almost 10% of patients who were treated with corticosteroids developed fungal infections [138]. However, a confirmatory trial (AlcHepNet) found a significantly greater 90-day survival rate in patients who were treated with 40 mg of prednisone daily (90%) for ≤28 days compared with those treated with anakinra for 14 days and zinc for 90 days (72%) [139]. This trial had two important differences compared with the DASH trial—pentoxifylline was not used due to lack of efficacy in other trials and corticosteroids were discontinued in patients who were predicted to be “non-responders” based on the Lille score at 7 days [139, 140]. No patients in the AlcHepNet study developed fungal infections, possibly due to a reduction in exposure to corticosteroids in “non-responders” [138, 140]. This trial emphasized the importance of using the 7-day Lille score (>0.45) as a stopping rule in patients without a favorable response to corticosteroids.
Antioxidant therapy
AH is associated with oxidative stress and thus, among rat models, the use of N-acetylcysteine (NAC) attenuates liver damage [141]. NAC combined with prednisone improved 1-month survival for severe AH patients [142]. However, there was no improvement in 6-month survival and, in other clinical trials, NAC alone or with prednisone also did not show 6-month improved survival among AH patients [142, 143]. Metadoxine, which is an antioxidant, has been shown to have survival benefits at 3 and 6 months as an adjuvant therapy to steroids or pentoxifylline [144]. Moreover, patients on metadoxine were shown to have greater abstinence post-treatment compared with patients who were not on metadoxine (74.5% vs 59.4%, P = 0.02) [145]. However, more robust clinical evidence is needed before this therapeutic option can be considered as mainstream AH therapy.
Granulocyte modification therapy
Granulocyte colony stimulating factor (G-CSF), which is a glycoprotein that stimulates bone marrow production of neutrophils and stem cell release, demonstrated a reduced risk of death at 90 days in AH patients in a meta-analysis of seven studies (driven by studies from Asia) that involved 396 patients [146]. However, these findings have not been reproduced consistently outside of Asia [146]. Granulocytapheresis (GCAP) has emerged as a promising intervention in severe AH treatment by targeting and removing activated neutrophils that are responsible for liver damage [147]. One study highlighted its potential by showing improved outcomes in patients with high white blood cell counts who were treated with GCAP [148]. Despite the optimistic initial results, the need for further research through case studies and randomized controlled trials (RCTs) is emphasized to conclusively determine the efficacy of GCAP in this context.
Fecal microbiota transplantation and probiotics
Given the potential role of gut dysbiosis in the development of AH, fecal microbiota transplantation (FMT) has been studied in severe AH. Preliminary human trials indicate that FMT can improve disease severity and survival rates [149]. IMM-124E, which is hyperimmune bovine colostrum that is enriched with anti-lipopolysaccharide IgG antibodies, has shown promise for alleviating liver injury in non-alcohol steatohepatitis patients by modulating natural killer and T-cell functions [150, 151]. Several trials have explored its benefits for AH (NCT02473341 and NCT01968382), including a notable Phase II placebo-controlled RCT that was focused on assessing the effectiveness of IMM-124E in this condition, which showed a reduction in circulating endotoxin levels. Lactobacillus rhamnosus GG (LGG), which is a probiotic, resulted in more rapid improvement of liver injury and reduced alcohol consumption in patients with moderate AH [152].
Other novel therapies
As for further novel therapies, selonsertib (GS-4997), which is an oral inhibitor of ASK-1, was evaluated in the treatment of severe AH due to its role in mediating apoptosis, cytokine signaling, and stellate cell activation [153–156]. A Phase 2 trial suggested that selonsertib with prednisone did not improve 28-day mortality compared with placebo plus prednisone [157]. A clinical trial that investigated emricasan (NCT01912404), which is a pan-caspase inhibitor that is known for its effectiveness in sepsis [158], was halted due to challenges in establishing a safe dosage for AH patients, primarily because of problematic pharmacokinetics and high blood levels of the drug. Despite promising results in improving survival among patients with cirrhosis, its applicability to severe AH remains uncertain, highlighting the need for further dose-ranging studies to explore its potential efficacy in AH treatment [159]. Interleukin-22 (IL-22) reduced chronic-binge-drinking-induced liver injuries in mice models [160, 161]. In patients with AH, an open-label trial showed that IL-22 improved MELD and Lille scores, reduced inflammatory markers, and improved 42-day survival [162]. Additional new studies with this compound are at the planning stage. In a Phase 2b multicenter, open-label study, 30 mg of larsucosterol, which is an epigenetic modulator that inhibits DNA methylation, was found to reduce the risk of mortality (41% reduction, P = 0.07) among AH patients [163]. Larsucosterol may now enter a Phase 3 trial with the goal of Food Drug Administration (FDA) approval in the near future.
Obeticholic acid use in patients with moderate AH (NCT02039219) was tested in a Phase 2 placebo RCT that was terminated due to a black-box warning from the Food and Drug Administration in 2018 regarding safety issues with higher doses in patients with more advanced liver disease (Child B or C or with prior hepatic decompensation) [164]. 3α, 7α, 11β-trihydroxy-6α-ethyl-5β-cholan-24-oic acid (INT 787), which is a farnesoid X receptor agonist, is currently being studied in a randomized double-blind controlled multicenter trial (NCT05639543) as a possible treatment for patients with severe AH.
Nutritional therapy
Similarly to ALD, nutritional management plays a key role in the treatment of AH patients [165]. AH patients require 1.2–1.5 g/kg of protein with 35–40 kcal/kg of total caloric intake every day [166]. A study among American Veteran Affairs patients with AH found that undernourished patients had higher morbidity and mortality—specifically, those patients who had <21.5 kcal/kg of caloric intake per day had higher infection and mortality rates at 6 months [167]. For patients with severe AH who cannot meet their nutritional needs through food alone, the administration of nutritional therapy is crucial for enhancing survival, reducing infection rates, improving liver function, and aiding encephalopathy healing [56, 168]. Proper nutrition can significantly curb infection among AH patients, which is one of the leading causes of death among AH patients [112, 169, 170]. In managing nutritional support for patients, various feeding methods are considered based on the severity of the condition and the patient’s ability to tolerate different types of feeding [165]. Oral intake is preferred whenever possible due to its benefits for gut health but, in cases in which oral intake is insufficient, enteral feeding through a nasogastric tube is recommended, as it provides the necessary nutrients while maintaining gut integrity [165]. Parenteral nutrition, delivered intravenously, is considered a last resort due to its higher risk of complications such as infections [165]. Each method is chosen based on the patient’s specific nutritional needs and overall health status [165].
Liver transplantation
In medical non-responders, liver transplantation (LT) has been established as a lifesaving treatment modality for patients with severe AH. In the past, ALD management patients would be listed for LT only after 6 months of abstinence. Recent studies have shown that early LT for carefully selected AH patients has yielded excellent outcomes, with 1-year survival ranging from 86% to 95% [171–173]. Several factors play a role in the careful selection of AH patients for early LT, including social support (e.g. spousal or family support), employment, education, legal history (e.g. driving under the influence), comorbid substance use disorder and psychiatric illness, and a prior history of AH [174–181]. Given that early LT is now widely practiced as a treatment for AH patients, several scoring systems, including the Sustained Alcohol Use After Liver Transplantation score [178], Harmful Alcohol Relapse After Liver Transplant (HALT) score [177], High-Risk Alcoholism Relapse score [179, 180], Alcohol Relapse Risk Assessment score [182], and one developed using artificial intelligence (AI) [183], have been used to assess the risk of post-transplant return to drinking, which is a key consideration during the LT candidate selection process. An important note is that the NIAAA definition of AH does not distinguish patients who may recover from those with decompensated advanced cirrhosis (i.e. unlikely to recover). Methods of distinguishing patients with recovery potential from those who are unlikely to recover (and therefore truly need LT) are needed. It is important to note that LT in these patients is often performed without reliable predictive tools and there is no way to confirm that a patient will recover after the transplant. Moreover, 1-year survival post-LT is not a reliable marker of success for patients with AH, as our clinical experience shows that even those who return to drinking are likely to survive for 12 months.
Abstinence from alcohol
After recovering from AH, patients require management of the underlying AUD to alleviate their symptoms and improve survival. Abstinence is key for survival in this patient population, with the study by Potts et al. [184] showing that, among AH patients, the overall 5-year survival was 32%. However, upon stratification of this cohort, AH patients who abstained from alcohol had a 5-year survival rate of 75%. Even though abstinence improves overall survival, it does not avoid the risk of patients’ developing progressive liver disease, with estimates showing that 18% of AH patients without cirrhosis eventually develop cirrhosis even with full abstinence [185, 186].
AC management
The management of AC mirrors the broader management of any patient with cirrhosis. A summary of this management is provided in Table 4, which includes the importance of statin therapy consideration. Recent findings suggest that statins, which are typically used for cardiovascular protection, offer significant benefits for cirrhosis patients, despite concerns about liver toxicity [187, 188]. Research indicates that statins can reduce fibrosis progression and decompensation rates, with a meta-analysis showing HRs of 0.55 for fibrosis progression and 0.54 for decompensation [189]. A significant study found that simvastatin reduced all-cause mortality to 9% compared with 22% in controls over 24 months, although benefits were not observed in more advanced cirrhosis (Child–Pugh C) [190]. Statins are generally being used for patients with Child–Pugh A cirrhosis, higher albumin levels, higher platelet levels, lower international normalized ratios, lower bilirubin levels, and no liver complications [191]. Statins should be avoided in patients with a prior history of sensitivity to statins and prior hepatotoxicity to statins [191]. Patients who are on statins, especially those with cirrhosis or impaired liver function, should be monitored for adverse events such as statin-related myopathy, which can range from mild muscle pain to severe rhabdomyolysis and potential liver injury [192]. Other risks include new-onset diabetes and gastro-intestinal symptoms [192]. Extra caution is advised for patients with advanced liver disease (Child–Pugh Class C) due to their higher vulnerability to these side effects [192]. Despite these promising outcomes, caution with statin use is advised in advanced cirrhosis due to potential safety concerns, including in decompensated cirrhosis and the risk of rhabdomyolysis [193]. The need for further large-scale RCTs is emphasized in order to clarify the role of statins in managing cirrhosis and preventing its complications, with the aim of leveraging affordable cost and broadening therapeutic potential. The ongoing Liver Cirrhosis Network clinical trial aims to address this need (NCT05740358).
Table 4.
Treatment options for patients with AC
| Treatment | Mechanism of action | Indications and evidence |
|---|---|---|
| Statins | Improve endothelial function; anti-inflammatory | Emerging evidence suggests potential benefits in portal hypertension and liver fibrosis management |
| Human Serum Albumin (HSA) | Oncotic and non-oncotic properties | Reduces renal failure and mortality in spontaneous bacterial peritonitis, type 1 HRS, and paracentesis-induced circulatory dysfunction; prolongs survival in uncomplicated ascites with diuretics |
| S-Adenosylmethionine (SAM) | Methyl donor in all methylation reactions and regulates glutathione synthesis | Did not outperform placebo in treating ALD, suggesting abstinence as a more effective liver function improvement method; currently Phase 2 trial underway |
| Caffeine/coffee | Antagonizes A2a adenosine receptor; anti-fibrotic | Inverse dose–response relationship with cirrhosis risk; associated with lower liver stiffness and reduced HCC risk |
| Alcohol cessation interventions | Reduce liver injury | Critical for alcohol-related liver disease; includes behavioral and pharmacotherapy (e.g. naltrexone) |
| Dietary and lifestyle modifications | Improve overall liver health | Sodium restriction for ascites, nutrition for malnutrition, and exercise to reduce sarcopenia |
AC = alcohol-associated cirrhosis.
Caffeine and other compounds that are found in coffee, such as polyphenols, may offer protective effects against liver fibrosis and cirrhosis, and a notable inverse relationship between coffee consumption and the risk of cirrhosis or its complications has been proven [194]. A meta-analysis also supported potential beneficial impact of coffee on reducing hepatocellular carcinoma risk, showing an RR of 0.55 (95% CI, 0.44–0.67) [195]. Human Serum Albumin (HSA) plays a crucial role in managing cirrhosis complications through its oncotic and non-oncotic properties [196]. Long-term HSA plus diuretics has been shown to extend survival in cirrhotic patients with ascites, emphasizing the therapeutic potential of HSA in cirrhosis management [197–201]. Finally, patients with AC require standard cirrhosis longitudinal management, including portal hypertensive complications (e.g. varices), HE, hepatocellular carcinoma surveillance, nonselective beta-blocker consideration for portal hypertension reduction, and immunizations.
While the above-mentioned therapies are not necessarily specific for AC, there are some importance nuances to managing patients with AC, such as alcohol use and emerging tailored therapies that merit more granular discussion. First, alcohol abstinence was associated with a reduced risk of mortality after 1.5 years (HR, 0.51; 95% CI, 0.33–0.81) specifically among AC patients [75]. Second, certain unique pharmacological treatment of AC is being studied. The impact of S-Adenosylmethionine (SAM) on ALD was assessed through a 24-week double-blind randomized placebo-controlled trial. Although SAM is known as a methyl donor in all methylation reactions and regulates glutathione synthesis [202], which is the primary cellular antioxidant, the study found no significant difference between SAM and placebo in improving liver function tests or liver histopathology scores. While SAM treatment increased serum SAM levels, indicating good absorption, it did not outperform placebo in treating ALD, suggesting abstinence as a more effective liver function improvement method [203]. However, despite these results, a multicenter randomized placebo-controlled trial of SAM in patients with AC is currently in Phase 2 and is underway, with a hypothesis that SAM will improve liver function in patients with ALD and reduce all-cause mortality compared with placebo (NCT04250259). Many patients use nutritional supplements such as milk thistle, which has been proposed to have benefits and increase patient survival [204]. However, other studies have not confirmed these findings [119]. Several actively enrolling clinical trials for the treatment of AC are underway; these trials include Profermin, which is a modulator of gut microbiota (NCT 03863730), and two trials on Cellgram-LC, which is autologous mesenchymal stem cell therapy (NCT04689152 and NCT05093881).
Conclusions
In conclusion, addressing the global challenge of ALD necessitates a comprehensive strategy that integrates the latest advances in individualized treatment plans and the critical role of lifestyle modifications, including reducing alcohol intake. While the prevalence of ALD, AH, and AC continues to rise, the development and implementation of ICM, alongside innovative pharmacological and supportive therapies, mark significant strides toward improving patient outcomes. This approach hinges on a multifaceted framework that balances medical interventions with the essential, yet proportionate, emphasis on lifestyle adjustments to mitigate disease progression. The collaborative efforts of healthcare providers, patients, and support networks are essential in navigating the complexities of ALD management, aiming to reduce the burden of the disease through a blend of scientific advancements and practical, patient-centered care strategies.
Authors’ Contributions
A.A.: manuscript concept and design; drafting of manuscript; T.G.C. and M.C.M.: manuscript concept and design; critical revision of manuscript for important intellectual content drafting; T.A.K.: critical revision of manuscript for important intellectual content. All authors approved the final version to be published.
Contributor Information
Ahmad Anouti, Division of Digestive and Liver Diseases, UT Southwestern Medical Center, Dallas, TX, USA.
Thomas A Kerr, Division of Digestive and Liver Diseases, UT Southwestern Medical Center, Dallas, TX, USA.
Mack C Mitchell, Division of Digestive and Liver Diseases, UT Southwestern Medical Center, Dallas, TX, USA.
Thomas G Cotter, Division of Digestive and Liver Diseases, UT Southwestern Medical Center, Dallas, TX, USA.
Funding
T.G.C. is supported by the American Association for the Study of Liver Diseases (AASLD) Clinical, Translational and Outcomes Research Award (CTORA) and National Institute on Alcohol Abuse and Alcoholism (NIAAA) K23AA031310 grant. Research reported in this publication was supported by the NIAAA of the National Institutes of Health (NIH) under Award Number K23AA031310. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. M.C.M. is supported by a grant from the National Institute on Alcohol Abuse and Alcoholism U01 AA026975 and consults for GlaxoSmithKline, Amygdala NeProdigy, HepaTX, and Parvus. T.A.K. is supported by U01 AA026975 and has a research relationship with GSK and consults for Alexion.
Conflicts of Interest
Dr Anouti has no relevant disclosures.
References
- 1. Mellinger JL. Epidemiology of alcohol use and alcoholic liver disease. Clin Liver Dis (Hoboken) 2019;13:136–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang DQ, Mathurin P, Cortez-Pinto H. et al. Global epidemiology of alcohol-associated cirrhosis and HCC: trends, projections and risk factors. Nat Rev Gastroenterol Hepatol 2023;20:37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Osna NA, Donohue TM Jr, Kharbanda KK.. Alcoholic liver disease: pathogenesis and current management. Alcohol Res 2017;38:147–61. [PMC free article] [PubMed] [Google Scholar]
- 5. Rinella ME, Lazarus JV, Ratziu V. et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023;78:1966–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kalinowski A, Humphreys K.. Governmental standard drink definitions and low-risk alcohol consumption guidelines in 37 countries. Addiction 2016;111:1293–8. [DOI] [PubMed] [Google Scholar]
- 7. NIAAA. What is Heavy Drinking? 02/27/2024. https://www.niaaa.nih.gov/health-professionals-communities/core-resource-on-alcohol/basics-defining-how-much-alcohol-too-much#pub-toc4 (15 April 2024, date last accessed).
- 8. Hasin DS, O'Brien CP, Auriacombe M. et al. DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry 2013;170:834–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singal AK, Leggio L, DiMartini A.. Alcohol use disorder in alcohol-associated liver disease: two sides of the same coin. Liver Transpl 2024;30:200–12. [DOI] [PubMed] [Google Scholar]
- 10. Meza V, Arnold J, Díaz LA. et al. Alcohol Consumption: Medical Implications, the Liver and Beyond. A&A 2022;57:283–91. [DOI] [PubMed] [Google Scholar]
- 11. SAMHSA; Center for Behavioral Health Statistics and Quality. 2022 National Survey on Drug Use and Health Table 5.9A—Alcohol Use Disorder in Past Year: Among People Aged 12 Or Older; By Age Group and Demographic Characteristics, Numbers in Thousands, 2021 and 2022. https://www.samhsa.gov/data/sites/default/files/reports/rpt42728/NSDUHDetailedTabs2022/NSDUHDetailedTabs2022/NSDUHDetTabsSect5pe2022.htm#tab5.9a (15 April 2024, date last accessed).
- 12. SAMHSA; Center for Behavioral Health Statistics and Quality. 2022 National Survey on Drug Use and Health. Table 5.9B—Alcohol Use Disorder in Past Year: Among People Aged 12 Or Older; By Age Group and Demographic Characteristics, Percentages, 2021 and 2022. https://www.samhsa.gov/data/sites/default/files/reports/rpt42728/NSDUHDetailedTabs2022/NSDUHDetailedTabs2022/NSDUHDetTabsSect5pe2022.htm#tab5.9 (15 April 2024, date last accessed).
- 13. Anouti A, Mellinger JL.. The changing epidemiology of alcohol-associated liver disease: gender, race, and risk factors. Semin Liver Dis 2023;43:50–9. [DOI] [PubMed] [Google Scholar]
- 14. Mellinger J, Winder GS, Fernandez AC.. Measuring the alcohol in alcohol-associated liver disease: choices and challenges for clinical research. Hepatology 2021;73:1207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramkissoon R, Shah VH.. Alcohol use disorder and alcohol-associated liver disease. Alcohol Res 2022;42:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Niu X, Zhu L, Xu Y. et al. Global prevalence, incidence, and outcomes of alcohol related liver diseases: a systematic review and meta-analysis. BMC Public Health 2023;23:859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Singal AK, Kuo YF, Arab JP. et al. Racial and health disparities among cirrhosis-related hospitalizations in the USA. J Clin Transl Hepatol 2022;10:398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nguyen GC, Thuluvath PJ.. Racial disparity in liver disease: biological, cultural, or socioeconomic factors. Hepatology 2008;47:1058–66. [DOI] [PubMed] [Google Scholar]
- 19. Levy R, Catana AM, Durbin-Johnson B. et al. Ethnic differences in presentation and severity of alcoholic liver disease. Alcohol Clin Exp Res 2015;39:566–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han S, Yang Z, Zhang T. et al. Epidemiology of alcohol-associated liver disease. Clin Liver Dis 2021;25:483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Israelsen M, Torp N, Johansen S. et al. Validation of the new nomenclature of steatotic liver disease in patients with a history of excessive alcohol intake: an analysis of data from a prospective cohort study. Lancet Gastroenterol Hepatol 2024;9:218–8. [DOI] [PubMed] [Google Scholar]
- 22. Shroff H, Gallagher H.. Multidisciplinary care of alcohol-related liver disease and alcohol use disorder: a narrative review for hepatology and addiction clinicians. Clin Ther 2023;45:1177–88. [DOI] [PubMed] [Google Scholar]
- 23. Lackner C, Spindelboeck W, Haybaeck J. et al. Histological parameters and alcohol abstinence determine long-term prognosis in patients with alcoholic liver disease. J Hepatol 2017;66:610–8. [DOI] [PubMed] [Google Scholar]
- 24. Nischalke HD, Berger C, Luda C. et al. The PNPLA3 rs738409 148M/M genotype is a risk factor for liver cancer in alcoholic cirrhosis but shows no or weak association in hepatitis C cirrhosis. PLoS One 2011;6:e27087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burza MA, Molinaro A, Attilia ML. et al. PNPLA3 I148M (rs738409) genetic variant and age at onset of at‐risk alcohol consumption are independent risk factors for alcoholic cirrhosis. Liver Int 2014;34:514–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Friedrich K, Wannhoff A, Kattner S. et al. PNPLA3 in end‐stage liver disease: alcohol consumption, hepatocellular carcinoma development, and transplantation‐free survival. J Gastroenterol Hepatol 2014;29:1477–84. [DOI] [PubMed] [Google Scholar]
- 27. Chamorro AJ, Torres JL, Mirón‐Canelo JA. et al. Systematic review with meta‐analysis: the I148M variant of patatin‐like phospholipase domain‐containing 3 gene (PNPLA3) is significantly associated with alcoholic liver cirrhosis. Aliment Pharmacol Ther 2014;40:571–81. [DOI] [PubMed] [Google Scholar]
- 28. Kim HS, Xiao X, Byun J. et al. Synergistic Associations of PNPLA3 I148M Variant, Alcohol Intake, and Obesity With Risk of Cirrhosis, Hepatocellular Carcinoma, and Mortality. JAMA Netw Open 2022;5:e2234221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roerecke M, Vafaei A, Hasan OSM. et al. Alcohol consumption and risk of liver cirrhosis: a systematic review and meta-analysis. Am J Gastroenterol 2019;114:1574–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kubiliun MJ, Cohen JC, Hobbs HH. et al. Contribution of a genetic risk score to ethnic differences in fatty liver disease. Liver Int 2022;42:2227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Romeo S, Kozlitina J, Xing C. et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2008;40:1461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lazo M, Bilal U, Mitchell MC. et al. Interaction between alcohol consumption and PNPLA3 variant in the prevalence of hepatic steatosis in the US population. Clin Gastroenterol Hepatol 2021;19:2606–14.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Buch S, Stickel F, Trepo E. et al. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat Genet 2015;47:1443–8. [DOI] [PubMed] [Google Scholar]
- 34. Emdin CA, Haas M, Ajmera V. et al. Association of genetic variation with cirrhosis: a multi-trait genome-wide association and gene-environment interaction study. Gastroenterol 2021;160:1620–33.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang J, Trépo E, Nahon P. et al. A 17-beta-hydroxysteroid dehydrogenase 13 variant protects from hepatocellular carcinoma development in alcoholic liver disease. Hepatology 2019;70:231–40. [DOI] [PubMed] [Google Scholar]
- 36. Chen H, Zhang Y, Guo T. et al. Genetic variant rs72613567 of HSD17B13 gene reduces alcohol-related liver disease risk in Chinese Han population. Liver Int 2020;40:2194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kezer CA, Simonetto DA, Shah VH.. Sex differences in alcohol consumption and alcohol-associated liver disease. Mayo Clin Proc 2021;96:1006–16. [DOI] [PubMed] [Google Scholar]
- 38. Georgieva M, Xenodochidis C, Krasteva N.. Old age as a risk factor for liver diseases: Modern therapeutic approaches. Exp Gerontology 2023;184:112334. [DOI] [PubMed] [Google Scholar]
- 39. Israelsen M, Juel HB, Detlefsen S. et al. ; GALAXY and MicrobLiver consortiak. Metabolic and genetic risk factors are the strongest predictors of severity of alcohol-related liver fibrosis. Clin Gastroenterol Hepatol 2022;20:1784–94.e9. [DOI] [PubMed] [Google Scholar]
- 40. Åberg F, Puukka P, Sahlman P. et al. Metabolic risk factors for advanced liver disease among alcohol risk users in the general population. J Hepatol 2019;70:E273. [Google Scholar]
- 41. Pose E, Pera G, Torán P. et al. Interaction between metabolic syndrome and alcohol consumption, risk factors of liver fibrosis: a population‐based study. Liver Int 2021;41:1556–64. [DOI] [PubMed] [Google Scholar]
- 42. Whitfield JB, Schwantes-An TH, Darlay R. et al. ; GenomALC Consortium. A genetic risk score and diabetes predict development of alcohol-related cirrhosis in drinkers. J Hepatol 2022;76:275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hart CL, Morrison DS, Batty GD. et al. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. BMJ 2010;340:c1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bhadoria AS, Mishra S, Gawande K. et al. Personal or family history of metabolic traits predispose to higher hepatotoxic effects of alcohol. J Family Med Prim Care 2019;8:2558–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bhadoria AS, Kedarisetty CK, Bihari C. et al. Positive familial history for metabolic traits predisposes to early and more severe alcoholic cirrhosis: a cross-sectional study. Liver Int 2019;39:168–76. [DOI] [PubMed] [Google Scholar]
- 46. Ding C, Ng Fat L, Britton A. et al. Binge-pattern alcohol consumption and genetic risk as determinants of alcohol-related liver disease. Nat Commun 2023;14:8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vannier A, Fomin GL, Chung V. et al. Substance use disorder is associated with alcohol-associated liver disease in patients with alcohol use disorder. Gastro Hep Advances 2022;1:403–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Becker U, Deis A, Sorensen T. et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology 1996;23:1025–9. [DOI] [PubMed] [Google Scholar]
- 49. Ting PS, Lin WT, Huang CK. et al. Exclusive liquor and cocktail consumption is associated with at-risk fibrosis among nonheavy alcohol users with metabolic dysfunction-associated steatotic liver disease. Alcohol Clin Exp Res 2024;48:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sterling RK, Lissen E, Clumeck N. et al. ; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatol 2006;43:1317–25. [DOI] [PubMed] [Google Scholar]
- 51. Kim BK, Kim DY, Park JY. et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int 2010;30:546–53. [DOI] [PubMed] [Google Scholar]
- 52. McPherson S, Hardy T, Dufour JF. et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol 2017;112:740–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shinoda H, Watanabe Y, Fukai K. et al. Significance of Fib4 index as an indicator of alcoholic hepatotoxicity in health examinations among Japanese male workers: a cross-sectional and retrospectively longitudinal study. Eur J Med Res 2023;28:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Thiele M, Madsen BS, Hansen JF. et al. Accuracy of the enhanced liver fibrosis test vs fibrotest, elastography, and indirect markers in detection of advanced fibrosis in patients with alcoholic liver disease. Gastroenterology 2018;154:1369–79. [DOI] [PubMed] [Google Scholar]
- 55. Semmler G, Yang Z, Fritz L. et al. Dynamics in liver stiffness measurements predict outcomes in advanced chronic liver disease. Gastroenterol 2023;165:1041–52. [DOI] [PubMed] [Google Scholar]
- 56. Crabb DW, Im GY, Szabo G. et al. Diagnosis and treatment of alcohol-associated liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2020;71:306–33. [DOI] [PubMed] [Google Scholar]
- 57. Carithers JRL, Herlong HF, Diehl AM. et al. Methylprednisolone therapy in patients with severe alcoholic HepatitisA randomized multicenter trial. Ann Intern Med 1989;110:685–90. [DOI] [PubMed] [Google Scholar]
- 58. Maddrey WC, Boitnott JK, Bedine MS. et al. Corticosteroid therapy of alcoholic hepatitis. Gastroenterol 1978;75:193–9. [PubMed] [Google Scholar]
- 59. Soultati AS, Dourakis SP, Alexopoulou A. et al. Predicting utility of a model for end stage liver disease in alcoholic liver disease. WJG 2006;12:4020–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Owens RE, Snyder HS, Twilla JD. et al. Pharmacologic treatment of alcoholic hepatitis: examining outcomes based on disease severity stratification. J Clin Exp Hepatol 2016;6:275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rongey C, Kaplowitz N.. Current concepts and controversies in the treatment of alcoholic hepatitis. WJG 2006;12:6909–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vaa BE, Asrani SK, Dunn W. et al. Influence of serum sodium on MELD-based survival prediction in alcoholic hepatitis. Mayo Clin Proc 2011;86:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dominguez M, Rincón D, Abraldes JG. et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol 2008;103:2747–56. [DOI] [PubMed] [Google Scholar]
- 64. Mitri J, Almeqdadi M, Karagozian R.. Prognostic and diagnostic scoring models in acute alcohol-associated hepatitis: a review comparing the performance of different scoring systems. World J Hepatol 2023;15:954–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Forrest EH, Evans CD, Stewart S. et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut 2005;54:1174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Forrest EH, Morris AJ, Stewart S. et al. The Glasgow alcoholic hepatitis score identifies patients who may benefit from corticosteroids. Gut 2007;56:1743–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Morales-Arráez D, Ventura-Cots M, Altamirano J. et al. The MELD score is superior to the maddrey discriminant function score to predict short-term mortality in alcohol-associated hepatitis: a global study. Am J Gastroenterol 2022;117:301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fujiwara N, Trépo E, Raman I. et al. Plasma-signature-model for end-stage liver disease score to predict survival in severe alcoholic hepatitis. Clin Gastroenterol Hepatol 2022;20:651–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gonzalez-Quintela A, Garcia J, Campos J. et al. Serum cytokeratins in alcoholic liver disease: contrasting levels of cytokeratin-18 and cytokeratin-19. Alcohol 2006;38:45–9. [DOI] [PubMed] [Google Scholar]
- 70. Im GY. Emerging biomarkers in alcohol-associated hepatitis. J Clin Exp Hepatol 2023;13:103–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tornai D, Mitchell M, McClain CJ. et al. A novel score of IL-13 and age predicts 90-day mortality in severe alcohol-associated hepatitis: a multicenter plasma biomarker analysis. Hepatol Commun 2023;7:e0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dunn KE, Harrison JA, Leoutsakos J-M. et al. Continuous Abstinence During Early Alcohol Treatment is Significantly Associated with Positive Treatment Outcomes, Independent of Duration of Abstinence. Alcohol Alcohol 2017;52:72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Maisto SA, Clifford PR, Longabaugh R. et al. The relationship between abstinence for one year following pretreatment assessment and alcohol use and other functioning at two years in individuals presenting for alcohol treatment. J Stud Alcohol 2002;63:397–403. [DOI] [PubMed] [Google Scholar]
- 74. Hofer BS, Simbrunner B, Hartl L. et al. Alcohol abstinence improves prognosis across all stages of portal hypertension in alcohol-related cirrhosis. Clin Gastroenterol Hepatol 2023;21:2308–17.e7. [DOI] [PubMed] [Google Scholar]
- 75. Xie YD, Feng B, Gao Y. et al. Effect of abstinence from alcohol on survival of patients with alcoholic cirrhosis:a systematic review and meta-analysis. Hepatol Res 2014;44:436–49. [DOI] [PubMed] [Google Scholar]
- 76. Witkiewitz K, Wilson AD, Roos CR. et al. Can individuals with alcohol use disorder sustain non-abstinent recovery? non-abstinent outcomes 10 years after alcohol use disorder treatment. J Addict Med 2021;15:303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yang J, Kim S.. Factors affecting alcohol abstinence intentions of inpatients with alcohol use disorder. J Psychosoc Nurs Ment Health Serv 2021;59:23–32. [DOI] [PubMed] [Google Scholar]
- 78. Ware OD, Labos B, Hudgins D. et al. Prior periods of abstinence among adults with an alcohol use disorder: a qualitative template analysis. Subst Abuse 2023;17:11782218231162468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Henssler J, Müller M, Carreira H. et al. Controlled drinking-non-abstinent versus abstinent treatment goals in alcohol use disorder: a systematic review, meta-analysis and meta-regression. Addiction 2021;116:1973–87. [DOI] [PubMed] [Google Scholar]
- 80. Kelly JF, Humphreys K, Ferri M.. Alcoholics anonymous and other 12-step programs for alcohol use disorder . CDSR 2020;3:CD012880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jhanjee S. Evidence based psychosocial interventions in substance use. Indian J Psychol Med 2014;36:112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Weisner C, Matzger H, Kaskutas LA.. How important is treatment? One‐year outcomes of treated and untreated alcohol‐dependent individuals. Addiction 2003;98:901–11. [DOI] [PubMed] [Google Scholar]
- 83. Magill M, Apodaca TR, Borsari B. et al. A meta-analysis of motivational interviewing process: Technical, relational, and conditional process models of change. J Consult Clin Psychol 2018;86:140–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dieperink E, Fuller B, Isenhart C. et al. Efficacy of motivational enhancement therapy on alcohol use disorders in patients with chronic hepatitis C: a randomized controlled trial. Addiction 2014;109:1869–77. [DOI] [PubMed] [Google Scholar]
- 85. Miller WR, Rose GS.. Toward a theory of motivational interviewing. Am Psychol 2009;64:527–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. DeMartini KS, Schilsky ML, Palmer A. et al. Text messaging to reduce alcohol relapse in prelisting liver transplant candidates: a pilot feasibility study. Alcohol Clin Exp Res 2018;42:761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Suffoletto B, Scaglione S.. Using digital interventions to support individuals with alcohol use disorder and advanced liver disease: a bridge over troubled waters. Alcohol Clin Exp Res 2018;42:1160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Magill M, Bernstein MH, Hoadley A. et al. Do what you say and say what you are going to do: a preliminary meta-analysis of client change and sustain talk subtypes in motivational interviewing. Psychother Res 2019;29:860–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. McCrady BS, Owens MD, Borders AZ. et al. Psychosocial approaches to alcohol use disorders since 1940: a review. J Stud Alcohol Drugs Suppl 2014;75:68–78. [DOI] [PubMed] [Google Scholar]
- 90. Hemrage S, Brobbin E, Deluca P. et al. Efficacy of psychosocial interventions to reduce alcohol use in comorbid alcohol use disorder and alcohol-related liver disease: a systematic review of randomized controlled trials. Alcohol Alcohol 2023;58:478–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lee YK, Park SW, Kim YK. et al. Effects of naltrexone on the ethanol-induced changes in the rat central dopaminergic system. Alcohol Alcohol 2005;40:297–301. [DOI] [PubMed] [Google Scholar]
- 92. Harris BR, Prendergast MA, Gibson DA. et al. Acamprosate inhibits the binding and neurotoxic effects of trans-ACPD, suggesting a novel site of action at metabotropic glutamate receptors. Alcohol Clin Exp Res 2002;26:1779–93. [DOI] [PubMed] [Google Scholar]
- 93. Pedersen B, Askgaard G, Jørgensen C. et al. ; Cochrane Drugs and Alcohol Group. Disulfiram for alcohol use disorder. CDSR 2018;2018:CD010487. [Google Scholar]
- 94. Ramer L, Tihy M, Goossens N. et al. Disulfiram-induced acute liver injury. Case Rep Hepatol 2020;2020:8835647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hauser P, Fuller B, Ho SB. et al. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction 2017;112:1173–83. [DOI] [PubMed] [Google Scholar]
- 96. Paparrigopoulos T, Tzavellas E, Karaiskos D. et al. Treatment of alcohol dependence with low-dose topiramate: an open-label controlled study. BMC Psychiatry 2011;11:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Feinn R, Curtis B, Kranzler HR.. Balancing risk and benefit in heavy drinkers treated with topiramate: implications for personalized care. J Clin Psychiatry 2016;77:e278–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Anton RF, Latham P, Voronin K. et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med 2020;180:728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Anton RF, O’Malley SS, Ciraulo DA. et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 2006;295:2003–17. [DOI] [PubMed] [Google Scholar]
- 100. Diehl AM. Alcoholic liver disease: natural history. Liver Transplantation 1997;3:206–11. [PubMed] [Google Scholar]
- 101. Luca A, Garcia-Pagan JC, Bosch J. et al. Effects of ethanol consumption on hepatic hemodynamics in patients with alcoholic cirrhosis. Gastroenterol 1997;112:1284–9. [DOI] [PubMed] [Google Scholar]
- 102. Teli MR, Day CP, Burt AD. et al. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet 1995;346:987–90. [DOI] [PubMed] [Google Scholar]
- 103. DiMartini AF, Leggio L, Singal AK.. Barriers to the management of alcohol use disorder and alcohol-associated liver disease: strategies to implement integrated care models. Lancet Gastroenterol Hepatol 2022;7:186–95. [DOI] [PubMed] [Google Scholar]
- 104. Lucey MR, Singal AK.. Integrated treatment of alcohol use disorder in patients with alcohol‐associated liver disease: an evolving story. Hepatology 2020;71:1891–1893. [DOI] [PubMed] [Google Scholar]
- 105. Glance JB, DiMartini A.. Identification and management of alcohol dependence and withdrawal in alcoholic liver disease. Clin Liver Dis (Hoboken) 2013;2:57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Rogal S, Youk A, Zhang H. et al. Impact of alcohol use disorder treatment on clinical outcomes among patients with cirrhosis. Hepatology 2020;71:2080–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Caputo F, Domenicali M, Bernardi M.. Diagnosis and treatment of alcohol use disorder in patients with end‐stage alcoholic liver disease. Hepatol 2019;70:410–7. [DOI] [PubMed] [Google Scholar]
- 108. Leggio L, Lee MR.. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med 2017;130:124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Winder GS, Fernandez AC, Klevering K. et al. Confronting the crisis of comorbid alcohol use disorder and alcohol-related liver disease with a novel multidisciplinary clinic. Psychosomatics 2020;61:238–53. [DOI] [PubMed] [Google Scholar]
- 110. Sedarous M, Flemming JA.. Culture, stigma, and inequities creating barriers in alcohol use disorder management in alcohol-associated liver disease. Clin Liver Dis (Hoboken) 2023;21:130–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Jophlin LL, Singal AK, Bataller R. et al. ACG clinical guideline: alcohol-associated liver disease. Am J Gastroenterol 2024;119:30–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Thursz M, Gual A, Lackner C. et al. EASL Clinical Practice Guidelines: management of alcohol-related liver disease. J Hepatol 2018;69:154–81. [DOI] [PubMed] [Google Scholar]
- 113. Plank LD, Gane EJ, Peng S. et al. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: a randomized 12-month trial. Hepatology 2008;48:557–66. [DOI] [PubMed] [Google Scholar]
- 114. Kaur P, Verma N, Garg P. et al. Myokines are associated with progression, course and mortality in alcohol-associated liver disease. AP&T 2024;60:1005–20. [DOI] [PubMed] [Google Scholar]
- 115. Fialla AD, Israelsen M, Hamberg O. et al. Nutritional therapy in cirrhosis or alcoholic hepatitis: a systematic review and meta-analysis. Liver Int 2015;35:2072–8. [DOI] [PubMed] [Google Scholar]
- 116. AH Network. AlcHepNet. 2017. –2018. http://www.alchepnet.org/index.html (6 February 2024, date last accessed).
- 117. Taïeb J, Mathurin P, Elbim C. et al. Blood neutrophil functions and cytokine release in severe alcoholic hepatitis: effect of corticosteroids. J Hepatol 2000;32:579–86. [DOI] [PubMed] [Google Scholar]
- 118. Mathurin P, Duchatelle V, Ramond MJ. et al. Survival and prognostic factors in patients with severe alcoholic hepatitis treated with prednisolone. Gastroenterol 1996;110:1847–53. [DOI] [PubMed] [Google Scholar]
- 119. Rambaldi A, Saconato H, Christensen E. et al. Systematic review: glucocorticosteroids for alcoholic hepatitis–a Cochrane Hepato‐Biliary Group systematic review with meta‐analyses and trial sequential analyses of randomized clinical trials. Aliment Pharmacol Ther 2008;27:1167–78. [DOI] [PubMed] [Google Scholar]
- 120. Thursz MR, Richardson P, Allison M. et al. ; STOPAH Trial. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med 2015;372:1619–28. [DOI] [PubMed] [Google Scholar]
- 121. Louvet A, Thursz MR, Kim DJ. et al. Corticosteroids reduce risk of death within 28 days for patients with severe alcoholic hepatitis, compared with pentoxifylline or placebo-a meta-analysis of individual data from controlled trials. Gastroenterology 2018;155:458–68.e8. [DOI] [PubMed] [Google Scholar]
- 122. Louvet A, Naveau S, Abdelnour M. et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007;45:1348–54. [DOI] [PubMed] [Google Scholar]
- 123. Sandahl TD, Jepsen P, Ott P. et al. Validation of prognostic scores for clinical use in patients with alcoholic hepatitis. Scand J Gastroenterol 2011;46:1127–32. [DOI] [PubMed] [Google Scholar]
- 124. Garcia-Saenz-de-Sicilia M, Duvoor C, Altamirano J. et al. A day-4 lille model predicts response to corticosteroids and mortality in severe alcoholic hepatitis. Am J Gastroenterol 2017;112:306–15. [DOI] [PubMed] [Google Scholar]
- 125. Kamath PS, Therneau T, Shah VH.. MELDing the Lille score to more accurately predict mortality in alcoholic hepatitis. Gastroenterology 2015;149:281–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Foncea CG, Sporea I, Lupușoru R. et al. Day-4 Lille score is a good prognostic factor and early predictor in assessing therapy response in patients with liver cirrhosis and severe alcoholic hepatitis. J Clin Med 2021;10:2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Im GY, Lucey MR.. Practical concerns and controversies in the management of alcoholic hepatitis. Gastroenterol Hepatol (N Y) 2016;12:478–89. [PMC free article] [PubMed] [Google Scholar]
- 128. Felver ME, Mezey E, McGuire M. et al. Plasma tumor necrosis factor alpha predicts decreased long-term survival in severe alcoholic hepatitis. Alcoholism Clin Exp Res 1990;14:255–9. [DOI] [PubMed] [Google Scholar]
- 129. McHutchison J, Runyon B, Draguesku J. et al. Pentoxifylline may prevent renal impairment (hepatorenal-syndrome) in severe acute alcoholic hepatitis. Hepatology 1991;14:96. [Google Scholar]
- 130. Akriviadis E, Botla R, Briggs W. et al. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology 2000;119:1637–48. [DOI] [PubMed] [Google Scholar]
- 131. Naveau S, Chollet-Martin S, Dharancy S. et al. ; Foie-Alcool group of the Association Française pour l'Etude du Foie. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology 2004;39:1390–7. [DOI] [PubMed] [Google Scholar]
- 132. Blendis L, Dotan I.. Anti-TNF therapy for severe acute alcoholic hepatitis: what went wrong? Gastroenterology 2004;127:1637–9. [DOI] [PubMed] [Google Scholar]
- 133. Boetticher NC, Peine CJ, Kwo P. et al. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology 2008;135:1953–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Karakike E, Moreno C, Gustot T.. Infections in severe alcoholic hepatitis. Ann Gastroenterol 2017;30:152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Jiménez C, Ventura-Cots M, Sala M. et al. Effect of rifaximin on infections, acute-on-chronic liver failure and mortality in alcoholic hepatitis: a pilot study (RIFA-AH). Liver Int 2022;42:1109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Louvet A, Labreuche J, Dao T. et al. Effect of prophylactic antibiotics on mortality in severe alcohol-related hepatitis: a randomized clinical trial. JAMA 2023;329:1558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Petrasek J, Bala S, Csak T. et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest 2012;122:3476–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Szabo G, Mitchell M, McClain CJ. et al. IL-1 receptor antagonist plus pentoxifylline and zinc for severe alcohol-associated hepatitis. Hepatology 2022;76:1058–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Tu W, Gawrieh S, Dasarathy S. et al. ; Alcoholic Hepatitis Network (AlcHepNet) Investigators. Design of a multicenter randomized clinical trial for treatment of alcohol-associated hepatitis. Contemp Clin Trials Commun 2023;32:101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Gawrieh S, Dasarathy S, Tu W. et al. ; AlcHepNet Investigators. Randomized-controlled trial of anakinra plus zinc vs. prednisone for severe alcohol-associated hepatitis. J Hepatol 2024;80:684–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ozaras R, Tahan V, Aydin S. et al. N-acetylcysteine attenuates alcohol-induced oxidative stress in the rat. WJG 2003;9:125–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Nguyen-Khac E, Thevenot T, Piquet MA. et al. ; AAH-NAC Study Group. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med 2011;365:1781–9. [DOI] [PubMed] [Google Scholar]
- 143. Stewart S, Prince M, Bassendine M. et al. A randomized trial of antioxidant therapy alone or with corticosteroids in acute alcoholic hepatitis. J Hepatol 2007;47:277–83. [DOI] [PubMed] [Google Scholar]
- 144. Servín-Caamaño A, Cruz-Herrera J, Serralde-Zúñiga A. et al. Treatment with metadoxine and its impact on early mortality in patients with severe alcoholic hepatitis. Ann Hepatol 2014;13:343–52. [PubMed] [Google Scholar]
- 145. Higuera-de la Tijera F, Servín-Caamaño AI, Serralde-Zúñiga AE. et al. Metadoxine improves the three- and six-month survival rates in patients with severe alcoholic hepatitis. World J Gastroenterol 2015;21:4975–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Marot A, Singal AK, Moreno C. et al. Granulocyte colony-stimulating factor for alcoholic hepatitis: a systematic review and meta-analysis of randomised controlled trials. JHEP Rep 2020;2:100139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Watanabe Y, Kamimura K, Iwasaki T. et al. Case of severe alcoholic hepatitis treated with granulocytapheresis. World J Clin Cases 2016;4:369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Saniabadi AR, Hanai H, Takeuchi K. et al. Adacolumn, an adsorptive carrier based granulocyte and monocyteapheresis device for the treatment of inflammatory and refractory diseases associated with leukocytes. Ther Apher Dial 2003;7:48–59. [DOI] [PubMed] [Google Scholar]
- 149. Shasthry SM. Fecal microbiota transplantation in alcohol related liver diseases. Clin Mol Hepatol 2020;26:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Döhler JR, Nebermann L.. Bovine colostrum in oral treatment of enterogenic endotoxaemia in rats. Crit Car 2002;6:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Mizrahi M, Shabat Y, Ben Ya’acov A. et al. Alleviation of insulin resistance and liver damage by oral administration of Imm124-E is mediated by increased Tregs and associated with increased serum GLP-1 and adiponectin: results of a phase I/II clinical trial in NASH. J Inflamm Res 2012;5:141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Vatsalya V, Feng W, Kong M. et al. The beneficial effects of lactobacillus GG therapy on liver and drinking assessments in patients with moderate alcohol-associated hepatitis. Am J Gastroenterol 2023;118:1457–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Vallerie SN, Hotamisligil GS.. The role of JNK proteins in metabolism. Sci Transl Med 2010;2:60rv5. [DOI] [PubMed] [Google Scholar]
- 154. Mandrekar P, Ambade A, Lim A. et al. An essential role for monocyte chemoattractant protein‐1 in alcoholic liver injury: regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology 2011;54:2185–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Iracheta-Vellve A, Petrasek J, Satishchandran A. et al. Inhibition of sterile danger signals, uric acid and ATP, prevents inflammasome activation and protects from alcoholic steatohepatitis in mice. J Hepatol 2015;63:1147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Petrasek J, Iracheta-Vellve A, Saha B. et al. Metabolic danger signals, uric acid and ATP, mediate inflammatory cross-talk between hepatocytes and immune cells in alcoholic liver disease. J Leukoc Biol 2015;98:249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Mathurin P, Dufour J-F, Bzowej NH. et al. Selonsertib in combination with prednisolone for the treatment of severe alcoholic hepatitis: a phase 2 randomized controlled trial. Hepatology 2018;68:8A–9A. [Google Scholar]
- 158. Witek RP, Stone WC, Karaca FG. et al. Pan-caspase inhibitor VX-166 reduces fibrosis in an animal model of nonalcoholic steatohepatitis. Hepatology 2009;50:1421–30. [DOI] [PubMed] [Google Scholar]
- 159. Frenette CT, Morelli G, Shiffman ML. et al. Emricasan improves liver function in patients with cirrhosis and high model for end-stage liver disease scores compared with placebo. Clin Gastroenterol Hepatol 2019;17:774–83.e4. [DOI] [PubMed] [Google Scholar]
- 160. Ki SH, Park O, Zheng M. et al. Interleukin‐22 treatment ameliorates alcoholic liver injury in a murine model of chronic‐binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology 2010;52:1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Hendrikx T, Duan Y, Wang Y. et al. Bacteria engineered to produce IL-22 in intestine induce expression of REG3G to reduce ethanol-induced liver disease in mice. Gut 2019;68:1504–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Arab JP, Sehrawat TS, Simonetto DA. et al. An open‐label, dose‐escalation study to assess the safety and efficacy of IL‐22 agonist F‐652 in patients with alcohol‐associated hepatitis. Hepatology 2020;72:441–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Hassanein T, McClain CJ, Vatsalya V. et al. Safety, pharmacokinetics, and efficacy signals of larsucosterol (DUR-928) in alcohol-associated hepatitis. Am J Gastroenterol 2024;119:107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Peeraphatdit TB, Simonetto DA, Shah VH.. Exploring new treatment paradigms for alcoholic hepatitis by extrapolating from NASH and cholestasis. J Hepatol 2018;69:275–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Tadokoro T, Morishita A, Himoto T. et al. Nutritional support for alcoholic liver disease. Nutrients 2023;15:1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Plauth M, Cabre E, Riggio O. et al. ; ESPEN (European Society for Parenteral and Enteral Nutrition). ESPEN guidelines on enteral nutrition: liver disease. Clin Nutr 2006;25:285–94. [DOI] [PubMed] [Google Scholar]
- 167. Mendenhall CL, Moritz TE, Roselle GA. et al. ; THE VA COOPERATIVE STUDY GROUP #275. Protein energy malnutrition in severe alcoholic hepatitis: diagnosis and response to treatment. J Parenter Enteral Nutr 1995;19:258–65. [DOI] [PubMed] [Google Scholar]
- 168. Bischoff SC, Bernal W, Dasarathy S. et al. ESPEN practical guideline: clinical nutrition in liver disease. Clin Nutr 2020;39:3533–62. [DOI] [PubMed] [Google Scholar]
- 169. Trieu JA, Bilal M, Lewis B. et al. Adherence to appropriate nutrition in acute alcoholic hepatitis is low. Ann Hepatol 2018;17:752–5. [DOI] [PubMed] [Google Scholar]
- 170. Hosseini N, Shor J, Szabo G.. Alcoholic hepatitis: a review. Alcohol Alcohol 2019;54:408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Lee BP, Mehta N, Platt L. et al. Outcomes of early liver transplantation for patients with severe alcoholic hepatitis . Gastroenterology 2018;155:422–30.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Lee BP, Im GY, Rice JP. et al. Patterns of alcohol use after early liver transplantation for alcoholic hepatitis. Clin Gastroenterol Hepatol 2022;20:409–18.e5. [DOI] [PubMed] [Google Scholar]
- 173. Cotter TG, Sandıkçı B, Paul S. et al. Liver transplantation for alcoholic hepatitis in the United States: excellent outcomes with profound temporal and geographic variation in frequency. Am J Transplant 2021;21:1039–55. [DOI] [PubMed] [Google Scholar]
- 174. Volk ML, Biggins SW, Huang MA. et al. Decision making in liver transplant selection committees: a multicenter study. Ann Intern Med 2011;155:503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Guliyev C, İnce-Guliyev E, Ögel K.. Predictors of relapse to alcohol and substance use: are there any differences between 3 and 12 months after inpatient treatment? J Psychoactive Drugs 2022;54:358–67. [DOI] [PubMed] [Google Scholar]
- 176. Weinberg EM, Dukewich M, Jakhete N. et al. Early liver transplantation for severe alcohol-associated hepatitis and a history of prior liver decompensation. Am J Gastroenterol 2022;117:1990–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Satapathy SK, Thornburgh C, Heda R. et al. Predicting harmful alcohol relapse after liver transplant: the HALT score. Clin Transplant 2020;34:e14003. [DOI] [PubMed] [Google Scholar]
- 178. Lee BP, Vittinghoff E, Hsu C. et al. Predicting low risk for sustained alcohol use after early liver transplant for acute alcoholic hepatitis: the sustained alcohol use post-liver transplant score. Hepatology 2019;69:1477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Weeks SR, Sun Z, McCaul ME. et al. Liver transplantation for severe alcoholic hepatitis, updated lessons from the world's largest series. J Am Coll Surg 2018;226:549–57. [DOI] [PubMed] [Google Scholar]
- 180. De Gottardi A, Spahr L, Gelez P. et al. A simple score for predicting alcohol relapse after liver transplantation: results from 387 patients over 15 years. Arch Intern Med 2007;167:1183–8. [DOI] [PubMed] [Google Scholar]
- 181. Mathurin P, Moreno C, Samuel D. et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med 2011;365:1790–800. [DOI] [PubMed] [Google Scholar]
- 182. Rodrigue JR, Hanto DW, Curry MP.. The Alcohol Relapse Risk Assessment: a scoring system to predict the risk of relapse to any alcohol use after liver transplant. Prog Transpl 2013;23:310–8. [DOI] [PubMed] [Google Scholar]
- 183. Lee BP, Roth N, Rao P. et al. Artificial intelligence to identify harmful alcohol use after early liver transplant for alcohol-associated hepatitis. Am J Transplant 2022;22:1834–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Potts JR, Goubet S, Heneghan MA. et al. Determinants of long-term outcome in severe alcoholic hepatitis. Aliment Pharmacol Ther 2013;38:584–95. [DOI] [PubMed] [Google Scholar]
- 185. Galambos JT. Natural history of alcoholic hepatitis. 3. Histological changes. Gastroenterology 1972;63:1026–35. [PubMed] [Google Scholar]
- 186. Marbet UA, Bianchi L, Meury U. et al. Long-term histological evaluation of the natural history and prognostic factors of alcoholic liver disease. J Hepatol 1987;4:364–72. [DOI] [PubMed] [Google Scholar]
- 187. Vargas JI, Arrese M, Shah VH. et al. Use of statins in patients with chronic liver disease and cirrhosis: current views and prospects. Curr Gastroenterol Rep 2017;19:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Cabrera L, Abraldes JG.. Statins: the Panacea of Cirrhosis? Curr Hepatology Rep 2016;15:1–7. [Google Scholar]
- 189. Kamal S, Khan MA, Seth A. et al. Beneficial effects of statins on the rates of hepatic fibrosis, hepatic decompensation, and mortality in chronic liver disease: a systematic review and meta-analysis. Am J Gastroenterol 2017;112:1495–505. [DOI] [PubMed] [Google Scholar]
- 190. Abraldes JG, Villanueva C, Aracil C. et al. Addition of simvastatin to standard therapy for the prevention of variceal rebleeding does not reduce rebleeding but increases survival in patients with cirrhosis. Gastroenterology 2016;150:1160–70.e3. [DOI] [PubMed] [Google Scholar]
- 191. Kaplan DE. The use of statins in patients with cirrhosis. Gastroenterol Hepatol (N Y) 2018;14:485–7. [PMC free article] [PubMed] [Google Scholar]
- 192. Muñoz AE, Pollarsky FD, Marino M. et al. Addition of statins to the standard treatment in patients with cirrhosis: safety and efficacy. WJG 2021;27:4639–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Chalasani N, Younossi Z, Lavine JE. et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328–57. [DOI] [PubMed] [Google Scholar]
- 194. Corrao G, Zambon A, Bagnardi V. et al. ; Collaborative SIDECIR Group. Coffee, caffeine, and the risk of liver cirrhosis. Ann Epidemiol 2001;11:458–65. [DOI] [PubMed] [Google Scholar]
- 195. Yu C, Cao Q, Chen P. et al. An updated dose-response meta-analysis of coffee consumption and liver cancer risk. Sci Rep 2016;6:37488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Kockerling D, Nathwani R, Forlano R. et al. Current and future pharmacological therapies for managing cirrhosis and its complications. WJG 2019;25:888–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Arroyo V, García-Martinez R, Salvatella X.. Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol 2014;61:396–407. [DOI] [PubMed] [Google Scholar]
- 198. Garcia-Martinez R, Caraceni P, Bernardi M. et al. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology 2013;58:1836–46. [DOI] [PubMed] [Google Scholar]
- 199. Sanyal AJ, Boyer T, Garcia-Tsao G. et al. ; Terlipressin Study Group. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterol 2008;134:1360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Bernardi M, Caraceni P, Navickis RJ. et al. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology 2012;55:1172–81. [DOI] [PubMed] [Google Scholar]
- 201. Caraceni P, Riggio O, Angeli P. et al. ; ANSWER Study Investigators. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet 2018;391:2417–29. [DOI] [PubMed] [Google Scholar]
- 202. Lu SC. S-Adenosylmethionine. Int J Biochem Cell Biol 2000;32:391–5. [DOI] [PubMed] [Google Scholar]
- 203. Medici V, Virata MC, Peerson JM. et al. S-adenosyl-L-methionine treatment for alcoholic liver disease: a double-blinded, randomized, placebo-controlled trial. Alcohol Clin Exp Res 2011;35:1960–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Ferenci P, Dragosics B, Dittrich H. et al. Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J Hepatol 1989;9:105–13. [DOI] [PubMed] [Google Scholar]