Abstract
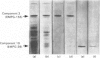
Two individual glycoprotein components from human milk-fat-globule membranes (MFGM) has been purified by selectively extracting the membrane glycoproteins followed by lectin affinity chromatography and gel filtration on Sephadex G-200 in the presence of protein-disaggregating agents. The purified glycoprotein components, termed 'epithelial-membrane glycoprotein' (EMGP-155 and EMGP-39) have estimated molecular weights of 155 000 and 39 000 respectively, and yield a single band under reducing conditions on sodium dodecyl sulphate/polyacrylamide gel. EMGP-155 and EMGP-39 contain 21.0% and 7.0% carbohydrate by weight, with fucose (13.5%, 12.4%), mannose (3.7%, 6.2%), galactose (28.5%, 22.6%), N-acetylglucosamine (17.8%, 7.4%) and sialic acid (36.4%, 51.4%) of the carbohydrate moiety respectively. For both the glycoprotein components, aspartic and glutamic acid and serine are the major amino acid residues.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M., Cawston T., Cheeseman G. C. Molecular-weight estimates of milk-fat-globule-membrane protein-sodium dodecyl sulphate complexes by electrophoresis in gradient acrylamide gels. Biochem J. 1974 Jun;139(3):653–660. doi: 10.1042/bj1390653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARGMANN W., FLEISCHHAUTER K., KNOOP A. [On the morphology of milk secretion. II. With a criticism of the scheme of secretion morphology]. Z Zellforsch Mikrosk Anat. 1961;53:545–568. [PubMed] [Google Scholar]
- Basch J. J., Farrell H. M., Greenberg R. Identification of the milk fat globule membrane proteins. I. Isolation and partial characterization of glycoprotein B. Biochim Biophys Acta. 1976 Nov 2;448(4):589–598. doi: 10.1016/0005-2736(76)90112-7. [DOI] [PubMed] [Google Scholar]
- Cawston T. E., Anderson M., Cheeseman G. C. Isolation, preparation and the amino acid composition of 4 milk-fat globule membrane proteins solubilized by treatment with sodium dodecyl sulphate. J Dairy Res. 1976 Oct;43(3):401–409. doi: 10.1017/s0022029900015983. [DOI] [PubMed] [Google Scholar]
- Farrar G. H., Harrison R. Isolation and structural characterization of alkali-labile oligosaccharides from bovine milk-fat-globule membrane. Biochem J. 1978 Jun 1;171(3):549–557. doi: 10.1042/bj1710549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenstein C., Keenan T. W., Eigel W. N., Sasaki M., Stadler J., Franke W. W. Preparation and characterization of the inner coat material associated with fat globule membranes from bovine and human milk. Exp Cell Res. 1979 Feb;118(2):277–294. doi: 10.1016/0014-4827(79)90153-8. [DOI] [PubMed] [Google Scholar]
- Harrison R., Higginbotham J. D., Newman R. Sialoglycopeptides from bovine milk fat globule membrane. Biochim Biophys Acta. 1975 May 21;389(3):449–463. doi: 10.1016/0005-2736(75)90156-x. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Hudson B. G., Wegener L. J., Wingate J. M., Carraway K. L. Chemical studies of erythrocyte membrane glycoproteins from several species. Comp Biochem Physiol B. 1975 May 15;51(1):127–135. doi: 10.1016/0305-0491(75)90370-3. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Cell surface proteins and malignant transformation. Biochim Biophys Acta. 1976 Apr 30;458(1):73–107. doi: 10.1016/0304-419x(76)90015-9. [DOI] [PubMed] [Google Scholar]
- Imam A., Laurence D. J., Neville A. M. Isolation and characterization of a major glycoprotein from milk-fat-globule membrane of human breast milk. Biochem J. 1981 Jan 1;193(1):47–54. doi: 10.1042/bj1930047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan T. W., Morré D. J., Olson D. E., Yunghans W. N., Patton S. Biochemical and morphological comparison of plasma membrane and milk fat globule membrane from bovine mammary gland. J Cell Biol. 1970 Jan;44(1):80–93. doi: 10.1083/jcb.44.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchen B. J. A comparison of the properties of membranes isolated from bovine skim milk and cream. Biochim Biophys Acta. 1974 Aug 9;356(3):257–269. doi: 10.1016/0005-2736(74)90266-1. [DOI] [PubMed] [Google Scholar]
- Kobylka D., Carraway K. L. Proteins and glycoproteins of the milk fat globule membrane. Biochim Biophys Acta. 1972 Nov 2;288(2):282–295. doi: 10.1016/0005-2736(72)90249-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Letarte-Muirhead M., Barclay A. N., Williams A. F. Purification of the Thy-1 molecule, a major cell-surface glycoprotein of rat thymocytes. Biochem J. 1975 Dec;151(3):685–697. doi: 10.1042/bj1510685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangino M. E., Brunner J. R. Molecular weight profile of fat globule membrane proteins. J Dairy Sci. 1975 Mar;58(3):313–318. doi: 10.3168/jds.s0022-0302(75)84567-x. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Andrews E. P. Glycoproteins: isolation from cellmembranes with lithium diiodosalicylate. Science. 1971 Dec 17;174(4015):1247–1248. doi: 10.1126/science.174.4015.1247. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Furthmayr H., Tomita M. The red cell membrane. Annu Rev Biochem. 1976;45:667–698. doi: 10.1146/annurev.bi.45.070176.003315. [DOI] [PubMed] [Google Scholar]
- Martel M. B., Dubois P., Got R. Membranes des globules lipidiques du lait humain. Preparation, etude morphologique et composition chimique. Biochim Biophys Acta. 1973 Jul 18;311(4):565–575. doi: 10.1016/0005-2736(73)90130-2. [DOI] [PubMed] [Google Scholar]
- Mather I. H., Keenan T. W. Studies on the structure of milk fat globule membrane. J Membr Biol. 1975 Apr 23;21(1-2):65–85. doi: 10.1007/BF01941062. [DOI] [PubMed] [Google Scholar]
- Roseman S. The synthesis of complex carbohydrates by multiglycosyltransferase systems and their potential function in intercellular adhesion. Chem Phys Lipids. 1970 Oct;5(1):270–297. doi: 10.1016/0009-3084(70)90024-1. [DOI] [PubMed] [Google Scholar]
- Snow L. D., Colton D. G., Carraway K. L. Purification and properties of the major sialoglycoprotein of the milk fat globule membrane. Arch Biochem Biophys. 1977 Mar;179(2):690–696. doi: 10.1016/0003-9861(77)90158-8. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Fairbanks G., Wallach D. F. Disposition of the major proteins in the isolated erythrocyte membrane. Proteolytic dissection. Biochemistry. 1971 Jun 22;10(13):2617–2624. doi: 10.1021/bi00789a031. [DOI] [PubMed] [Google Scholar]
- Wooding F. B. The structure of the milk fat globule membrane. J Ultrastruct Res. 1971 Nov;37(3):388–400. doi: 10.1016/s0022-5320(71)80133-8. [DOI] [PubMed] [Google Scholar]
- Woodruff J. J., Gesner B. M. The effect of neuraminidase on the fate of transfused lymphocytes. J Exp Med. 1969 Mar 1;129(3):551–567. doi: 10.1084/jem.129.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]