Abstract
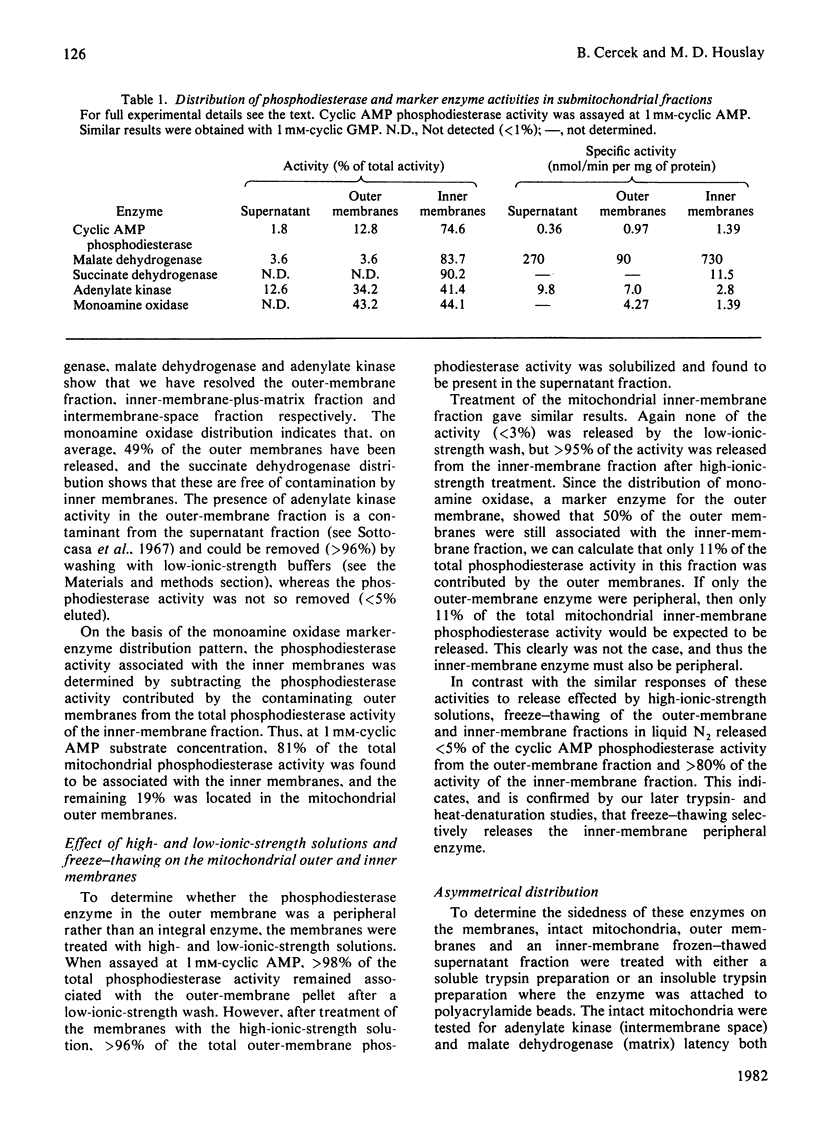
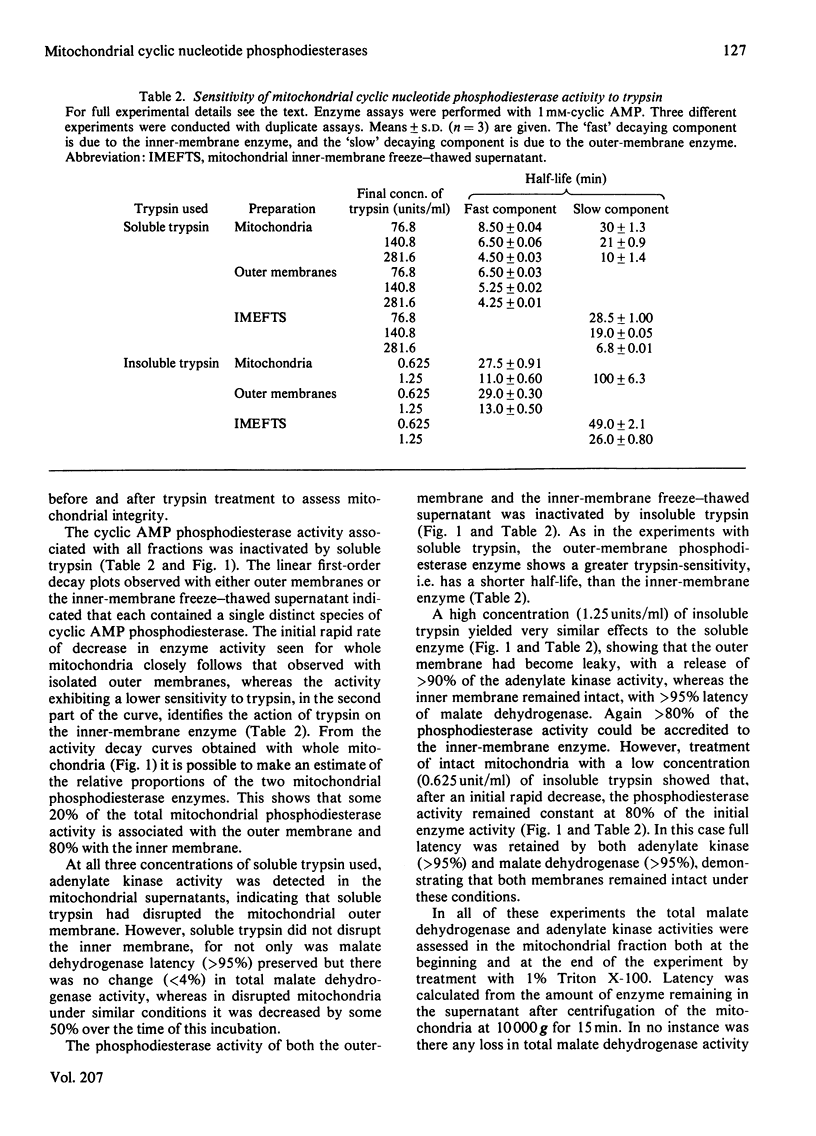
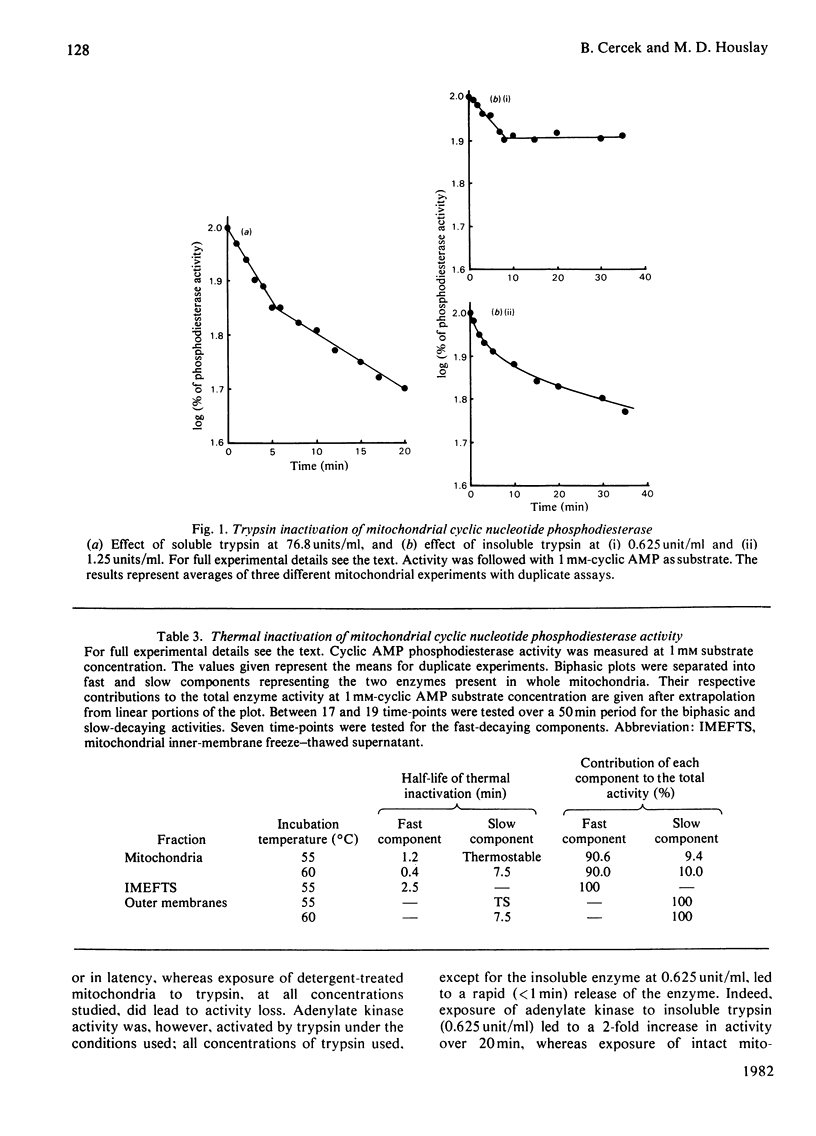
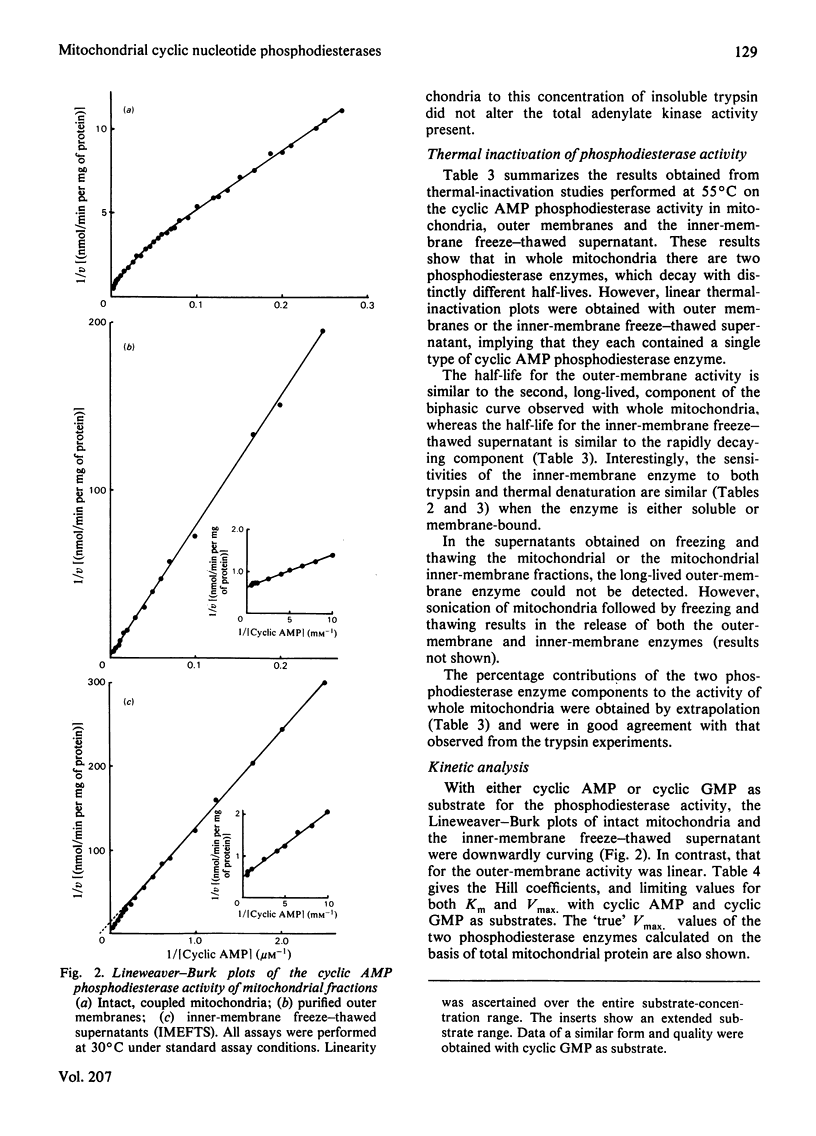
There are two distinct cyclic AMP phosphodiesterases associated with the liver mitochondrion: one with the outer membrane and one with the inner membrane. No activity is associated with the lysosomal fraction. Both of the enzymes are peripheral proteins and can be released from the membranes by high-ionic-strength treatment. Treatment of intact mitochondria with trypsin and insoluble trypsin localizes these enzymes to the cytosol-facing surface of their respective membranes. The enzymes differ in regard to sedimentation coefficient, thermostability and susceptibility to inactivation by trypsin. Both enzymes degrade cyclic AMP and cyclic GMP. Whereas the outer-membrane enzyme displays Michaelis kinetics and appears to be a low-affinity enzyme, the inner-membrane enzyme displays kinetics indicative of apparent negative co-operativity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleman M. M., Thompson W. J., Russell T. R. Cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1973;3:65–98. [PubMed] [Google Scholar]
- Arshad J. H., Holdsworth E. S. Stimulation of calcium efflux from rat liver mitochondria by adenosine 3'5 cyclic monophosphate. J Membr Biol. 1980 Dec 30;57(3):207–212. doi: 10.1007/BF01869588. [DOI] [PubMed] [Google Scholar]
- Bergeron J. J., Ehrenreich J. H., Siekevitz P., Palade G. E. Golgi fractions prepared from rat liver homogenates. II. Biochemical characterization. J Cell Biol. 1973 Oct;59(1):73–88. doi: 10.1083/jcb.59.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton L. L., Mayer S. E. Extrusion of cyclic AMP from pigeon erythrocytes. J Biol Chem. 1979 Oct 10;254(19):9714–9720. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. A simple and rapid assay of oxidative phosphorylation. Nature. 1955 Jun 25;175(4469):1120–1121. doi: 10.1038/1751120a0. [DOI] [PubMed] [Google Scholar]
- Erneux C., Boeynaems J. M., Dumont J. E. Theoretical analysis of the consequences of cyclic nucleotide phosphodiesterase negative co-operativity. Amplification and positive co-operativity of cyclic AMP accumulation. Biochem J. 1980 Oct 15;192(1):241–246. doi: 10.1042/bj1920241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell D. A. Theoretical analyses of the functioning of the high- and low-Km cyclic nucleotide phosphodiesterases in the regulation of the concentration of adenosine 3',5'-cyclic monophosphate in animal cells. J Theor Biol. 1980 May 21;84(2):361–385. doi: 10.1016/s0022-5193(80)80011-7. [DOI] [PubMed] [Google Scholar]
- Haslam R. J., Mills D. C. The adenylate kinase of human plasma, erythrocytes and platelets in relation to the degradation of adenosine diphosphate in plasma. Biochem J. 1967 Jun;103(3):773–784. doi: 10.1042/bj1030773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay M. D. Lipid substitution of mitochondrial monoamine oxidase can lead to the abolition of clorgyline selective inhibition without alteration in the A/B ratio assessed by substrate utilisation. Biochem Pharmacol. 1980 Dec 1;29(23):3211–3213. doi: 10.1016/0006-2952(80)90589-4. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Marchmont R. J. The insulin-stimulated cyclic AMP phosphodiesterase binds to a single class of protein sites on the liver plasma membrane. Biochem J. 1981 Sep 15;198(3):703–706. doi: 10.1042/bj1980703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay M. D. Membrane phosphorylation: a crucial role in the action of insulin, EGF, and pp60src? Biosci Rep. 1981 Jan;1(1):19–34. doi: 10.1007/BF01115146. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Palmer R. W. Changes in the form of Arrhenius plots of the activity of glucagon-stimulated adenylate cyclase and other hamster liver plasma-membrane enzymes occurring on hibernation. Biochem J. 1978 Sep 15;174(3):909–919. doi: 10.1042/bj1740909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Krinks M. H. Subunit structure and catalytic properties of bovine brain Ca2+-dependent cyclic nucleotide phosphodiesterase. Biochemistry. 1979 Feb 20;18(4):722–729. doi: 10.1021/bi00571a026. [DOI] [PubMed] [Google Scholar]
- Loten E. G., Assimacopoulos-Jeannet F. D., Exton J. H., Park C. R. Stimulation of a low Km phosphodiesterase from liver by insulin and glucagon. J Biol Chem. 1978 Feb 10;253(3):746–757. [PubMed] [Google Scholar]
- Marchmont R. J., Ayad S. R., Houslay M. D. Purification and properties of the insulin-stimulated cyclic AMP phosphodiesterase from rat liver plasma membranes. Biochem J. 1981 Jun 1;195(3):645–652. doi: 10.1042/bj1950645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchmont R. J., Houslay M. D. A peripheral and an intrinsic enzyme constitute the cyclic AMP phosphodiesterase activity of rat liver plasma membranes. Biochem J. 1980 May 1;187(2):381–392. doi: 10.1042/bj1870381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchmont R. J., Houslay M. D. Characterization of the phosphorylated form of the insulin-stimulated cyclic AMP phosphodiesterase from rat liver plasma membranes. Biochem J. 1981 Jun 1;195(3):653–660. doi: 10.1042/bj1950653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchmont R. J., Houslay M. D. Insulin trigger, cyclic AMP-dependent activation and phosphorylation of a plasma membrane cyclic AMP phosphodiesterase. Nature. 1980 Aug 28;286(5776):904–906. doi: 10.1038/286904a0. [DOI] [PubMed] [Google Scholar]
- Morrill M. E., Thompson S. T., Stellwagen E. Purification of a cyclic nucleotide phosphodiesterase from bovine brain using blue dextran-Sepharose chromatography. J Biol Chem. 1979 Jun 10;254(11):4371–4374. [PubMed] [Google Scholar]
- Pichard A. L., Cheung W. Y. Cyclid 3':5'-nucleotide phosphodiesterase. Interconvertible multiple forms and their effects on enzyme activity and kinetics. J Biol Chem. 1976 Sep 25;251(18):5726–5737. [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Biochemical properties of hormone-sensitive adenylate cyclase. Annu Rev Biochem. 1980;49:533–564. doi: 10.1146/annurev.bi.49.070180.002533. [DOI] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J. N., Hardman J. G. Cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1977;8:119–143. [PubMed] [Google Scholar]


