Abstract
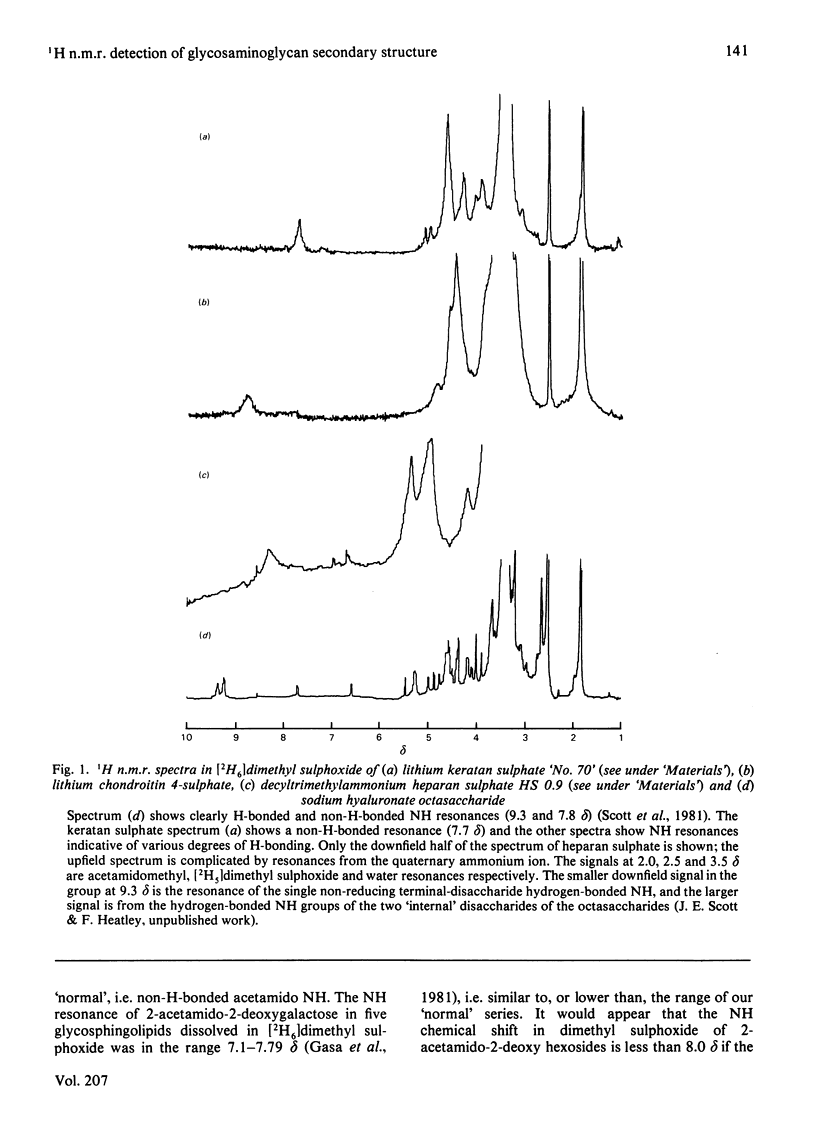
Two simple methods for dissolving salts of acid glycosaminoglycans with inorganic cations (e.g. Li+ and Na+) in dry dimethyl sulphoxide are described. Complete n.m.r. spectra of, e.g., Na+ and Li+ salts of chondroitin sulphate and keratan sulphate were obtained on these solutions. In [2H6]dimethyl sulphoxide the NH resonance of 2-acetamido-2-deoxy hexosides is in the range 7.2-8.0 delta, but is downfield (8.3-9.3 delta) when the NH is H-bonded to -CO2-. Heparan sulphate shows two NH resonances, of which one (at 8.3 delta) is probably indicative of H-bonding. Space-filling models show that a very close approach of NH to -CO2- across the alpha-glucosaminidic bond is possible, and a solution configuration for heparan sulphate is proposed. The n.m.r. results are entirely compatible with interpretations of periodate-oxidation kinetics, based on H-bonded secondary structures present in hyaluronate and chondroitin sulphates, but not in dermatan (or keratan) sulphate.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins E. D., Laurent T. C. X-ray-diffraction patterns from chondroitin 4-sulphate, dermatan sulphate and heparan sulphate. Biochem J. 1973 Jul;133(3):605–606. doi: 10.1042/bj1330605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIDSON E., HOFFMAN P., LINKER A., MEYER K. The acid mucopolysaccharides of connective tissue. Biochim Biophys Acta. 1956 Sep;21(3):506–518. doi: 10.1016/0006-3002(56)90188-3. [DOI] [PubMed] [Google Scholar]
- Fransson L. A. Interaction between dermatan sulphate chains. I. Affinity chromatography of copolymeric galactosaminioglycans on dermatan sulphate-substituted agarose. Biochim Biophys Acta. 1976 Jun 23;437(1):106–115. doi: 10.1016/0304-4165(76)90351-2. [DOI] [PubMed] [Google Scholar]
- Fransson L. A., Nieduszynski L. A., Sheehan J. K. Interaction between heparan sulphate chains. I. A gel chromatographic, light-scattering and structural study of aggregating and non-aggregating chains. Biochim Biophys Acta. 1980 Jun 19;630(2):287–300. doi: 10.1016/0304-4165(80)90433-x. [DOI] [PubMed] [Google Scholar]
- Linker A., Hovingh P. The heparitin sulfates (heparan sulfates). Carbohydr Res. 1973 Jul;29(1):41–62. doi: 10.1016/s0008-6215(00)82069-8. [DOI] [PubMed] [Google Scholar]
- SCOTT J. E. Aliphatic ammonium salts in the assay of acidic polysaccharides from tissues. Methods Biochem Anal. 1960;8:145–197. doi: 10.1002/9780470110249.ch4. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Heatley F. 1H nuclear-magnetic-resonance spectra of the methyl group of the acetamido moiety and the structure of acid glycosaminoglycans in solution. Biochem J. 1979 Aug 1;181(2):445–449. doi: 10.1042/bj1810445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Heatley F., Moorcroft D., Olavesen A. H. Secondary structures of hyaluronate and chondroitin sulphates. A 1H n.m.r. study of NH signals in dimethyl sulphoxide solution. Biochem J. 1981 Dec 1;199(3):829–832. doi: 10.1042/bj1990829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Tigwell M. J. Periodate oxidation and the shapes of glycosaminoglycuronans in solution. Biochem J. 1978 Jul 1;173(1):103–114. doi: 10.1042/bj1730103. [DOI] [PMC free article] [PubMed] [Google Scholar]



