Abstract
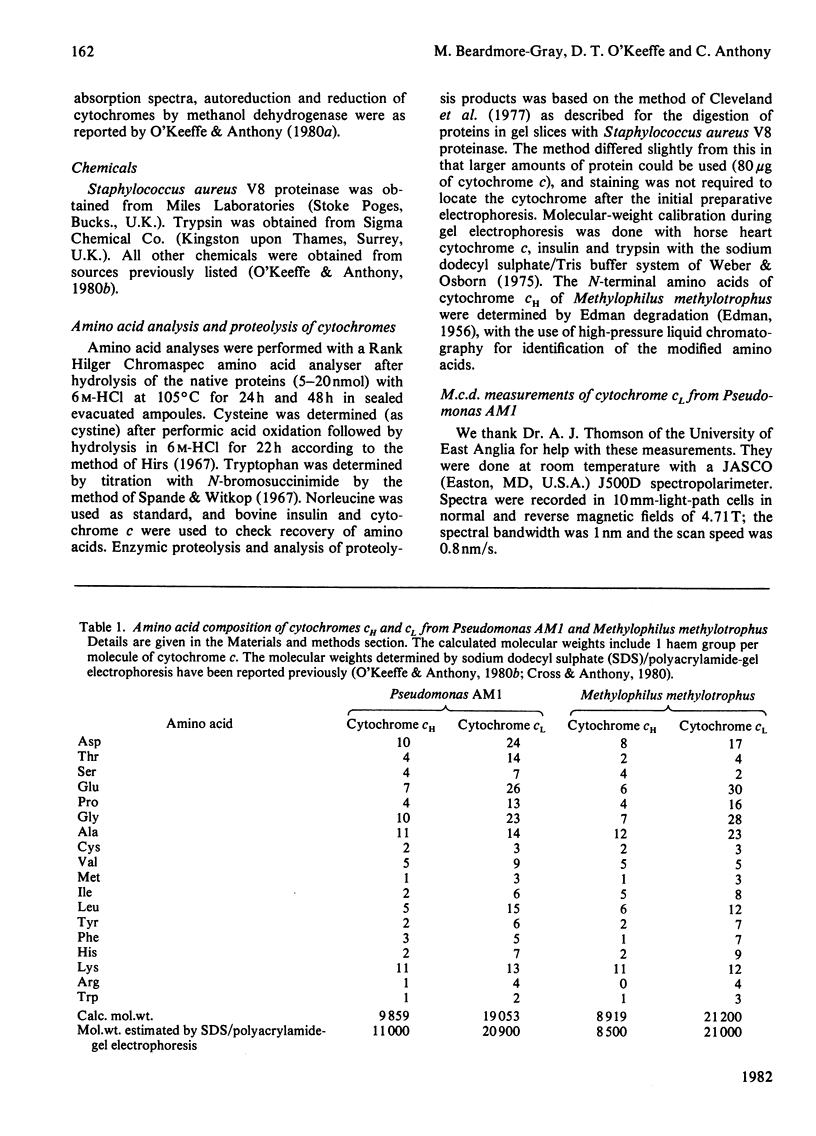
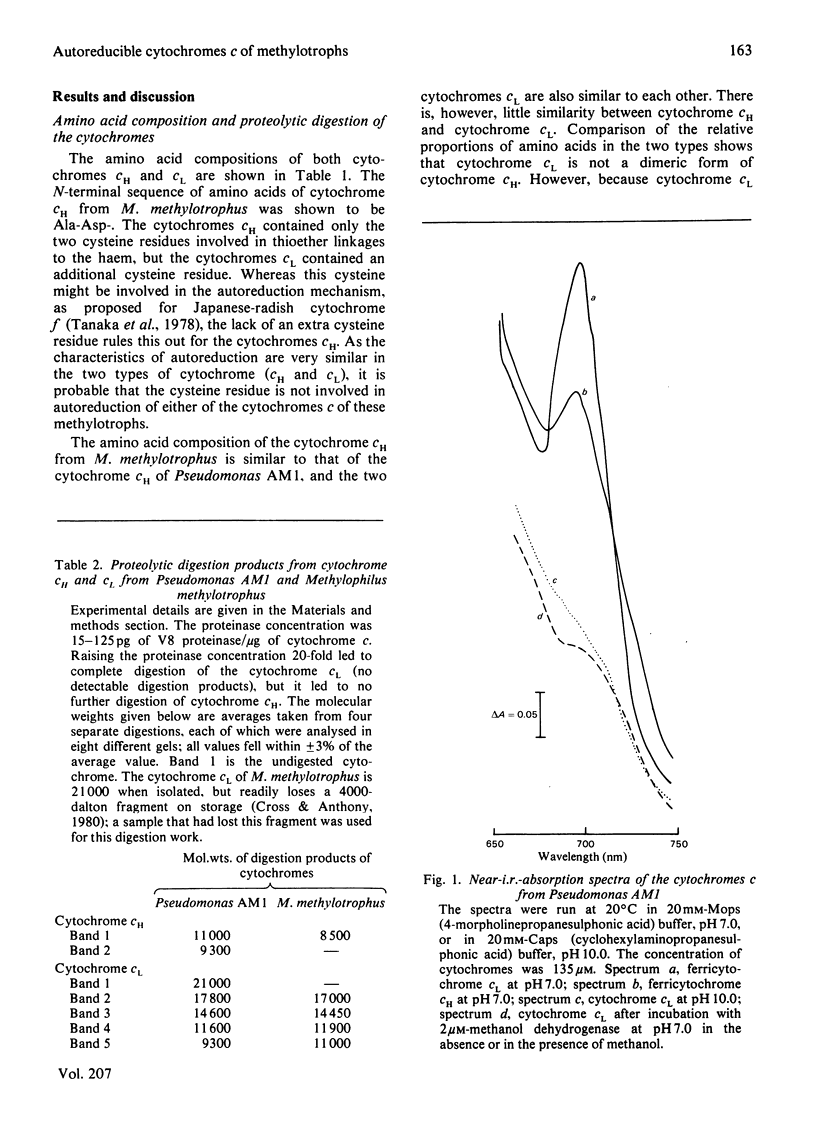
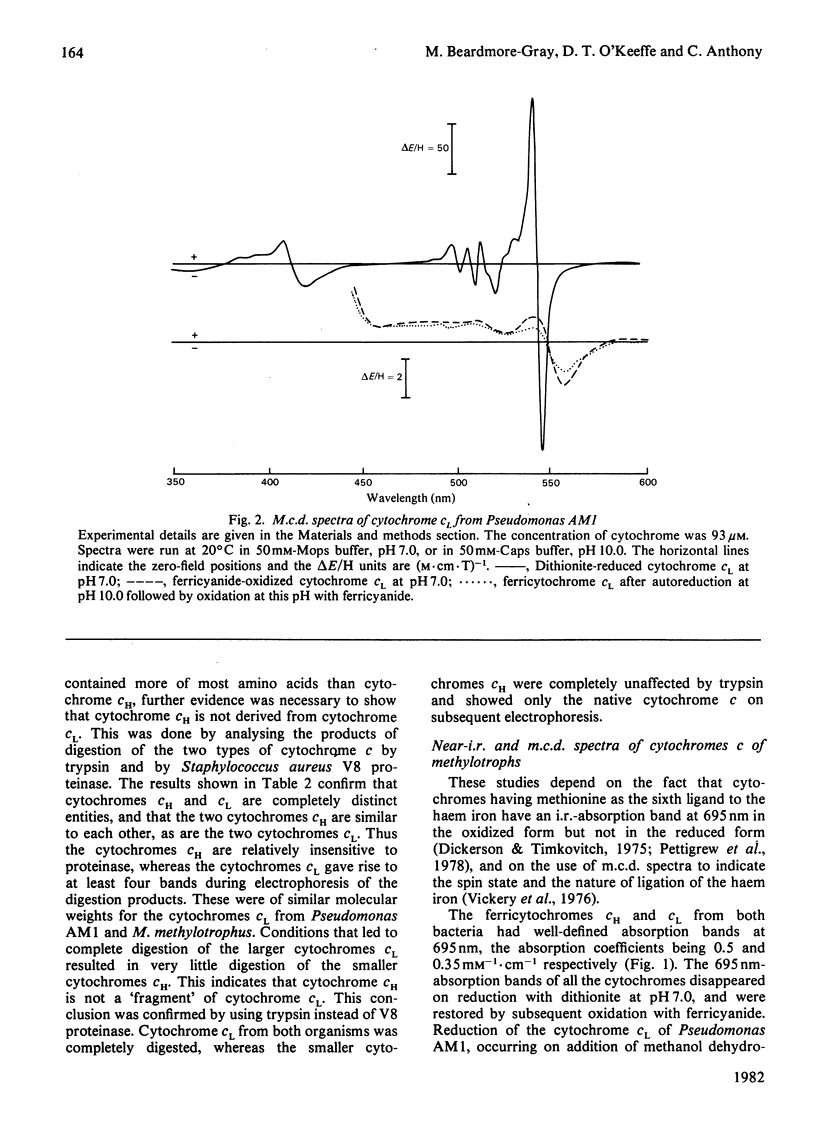
The two types of soluble cytochrome c (cytochrome cH and cytochrome cL) found in methylotrophs are completely distinct proteins; one type is not a dimer or degradation product of the other. Free thiol groups are probably not involved in the unusually rapid autoreduction of the cytochromes at high pH. The axial ligands to the haem iron, histidine and methionine, are the same as in other low-spin cytochromes c. The methionine ligand is displaced at high pH by an alternative strong-field ligand. This displacement does not occur on reduction of cytochrome cL by methanol dehydrogenase, but this does not rule out the possibility that the autoreduction mechanism is involved in the interaction of the dehydrogenase and cytochrome c.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cross A. R., Anthony C. The purification and properties of the soluble cytochromes c of the obligate methylotroph Methylophilus methylotrophus. Biochem J. 1980 Nov 15;192(2):421–427. doi: 10.1042/bj1920421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keeffe D. T., Anthony C. The interaction between methanol dehydrogenase and the autoreducible cytochromes c of the facultative methylotroph Pseudomonas AM1. Biochem J. 1980 Aug 15;190(2):481–484. doi: 10.1042/bj1900481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keeffe D. T., Anthony C. The two cytochromes c in the facultative methylotroph Pseudomonas am1. Biochem J. 1980 Nov 15;192(2):411–419. doi: 10.1042/bj1920411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta S., Tobari J. Two cytochromes c of Methylomonas J. J Biochem. 1981 Jul;90(1):215–224. doi: 10.1093/oxfordjournals.jbchem.a133452. [DOI] [PubMed] [Google Scholar]
- Pettigrew G. W., Bartsch R. G., Meyer T. E., Kamen M. D. Redox potentials of the photosynthetic bacterial cytochromes c2 and the structural bases for variability. Biochim Biophys Acta. 1978 Sep 7;503(3):509–523. doi: 10.1016/0005-2728(78)90150-0. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Takahashi M. A., Asada K. Isolation of monomeric cytochrome f from Japanese radish and a mechanism of autoreduction. J Biol Chem. 1978 Oct 25;253(20):7397–7403. [PubMed] [Google Scholar]
- Vickery L., Nozawa T., Sauer K. Magnetic circular dichroism studies of low-spin cytochromes. Temperature dependence and effects of axial coordination on the spectra of cytochrome c and cytochrome b5. J Am Chem Soc. 1976 Jan 21;98(2):351–357. doi: 10.1021/ja00418a006. [DOI] [PubMed] [Google Scholar]


