Abstract
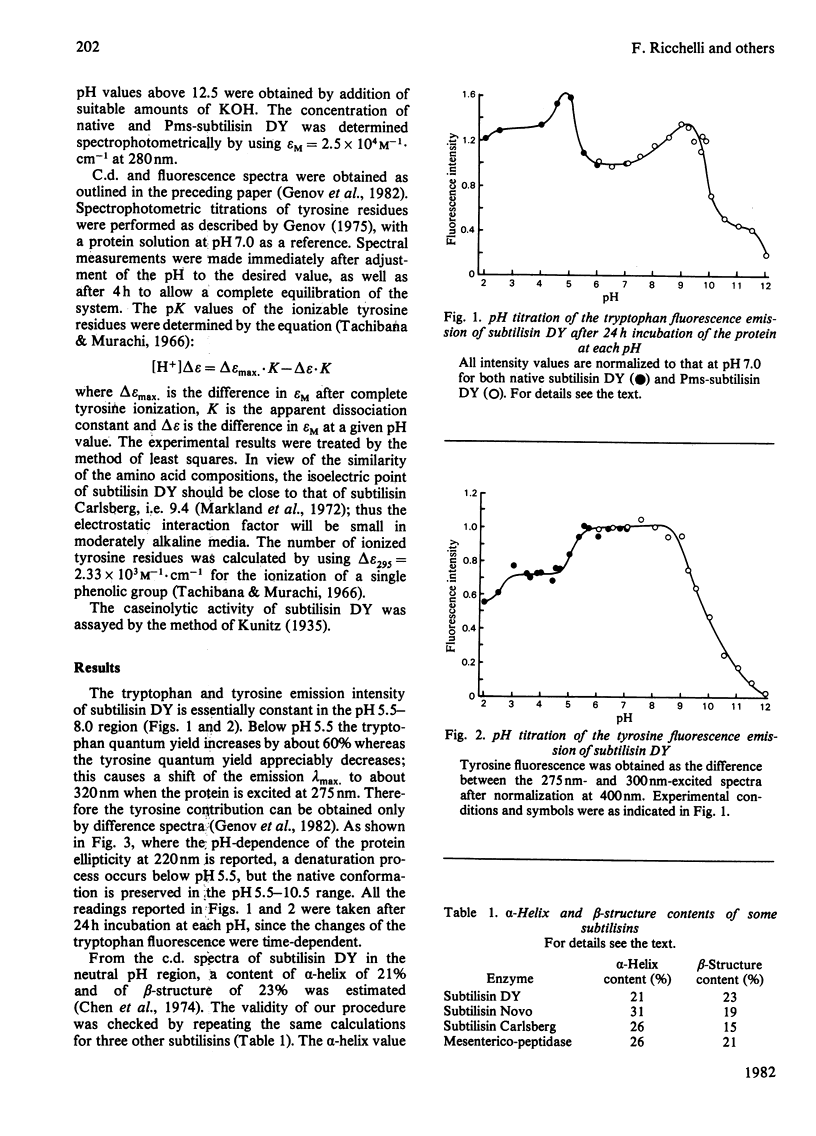
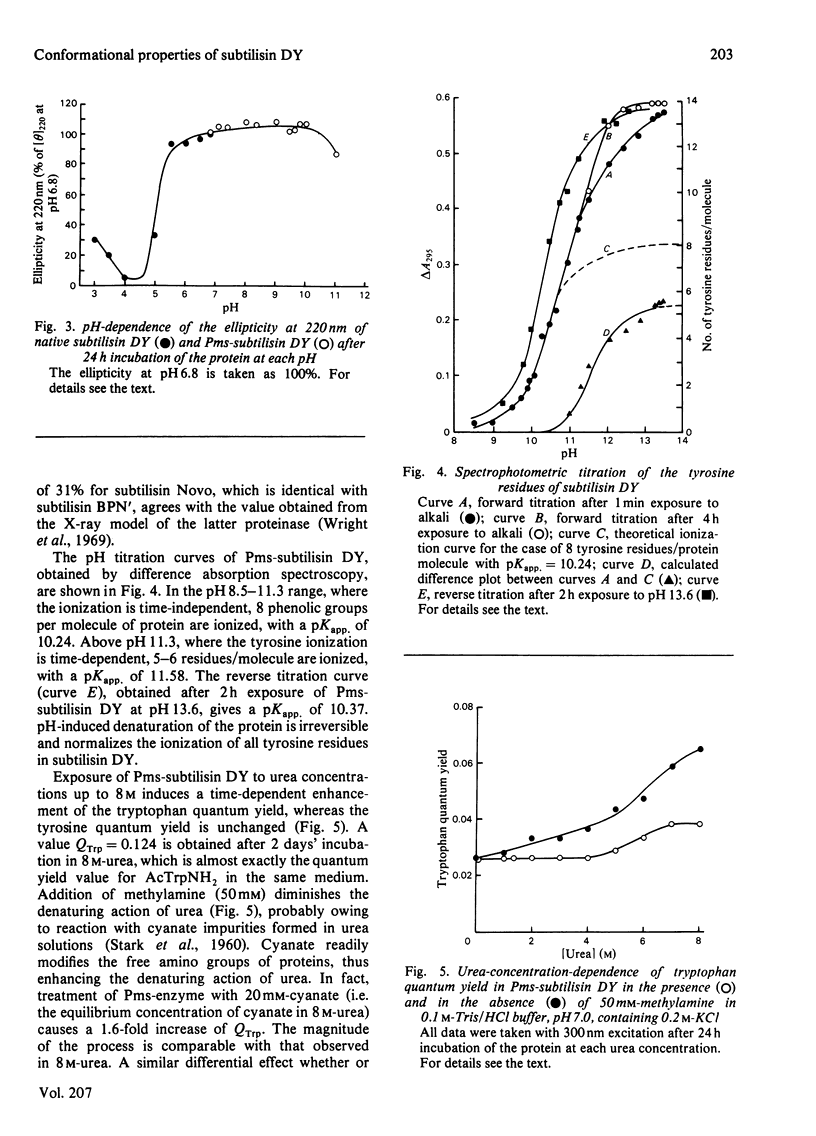
Subtilisin DY is very resistant to the denaturing action of urea: the conformational properties are not affected up to 4.5 M-urea, and even in the presence of 8 M-urea there is only a slow loss of ordered structure and caseinolytic activity. C.d. and fluorescence-emission studies also show that this proteinase is stable in the 5.5-10.0 pH range, whereas below pH 5.5 a sharp denaturation occurs that is complete at pH 4.5. Protein denaturation leads to a change of the emission quantum yield; in particular, in the native protein, indole fluorescence is quenched by some amino groups. Moreover, subtilisin DY possesses two classes of tyrosine residues: one class of exposed residues titrates normally, with pKapp. = 10.24, whereas one class of partially buried or hydrogen-bonded residues ionizes with pKapp. = 11.58. In general, such conformational properties resemble those of other subtilisins. However, some differences occur: e.g., subtilisin DY is less stable at acidic pH values and its tyrosine residues are more accessible to the solvent. Such differences are probably due to small variations of the three-dimensional structure; e.g., subtilisin DY has a slightly lower alpha-helix content.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. F., Omar S., Raubach R. A., Schleich T. Quenching of the tyrosyl and tryptophyl fluorescence of subtilisins Carlsberg and Novo by iodide. Biochemistry. 1977 Mar 8;16(5):987–992. doi: 10.1021/bi00624a028. [DOI] [PubMed] [Google Scholar]
- Brown M. F., Schleich T. Circular dichroism and gel filtration behavior of subtilisin enzymes in concentrated solutions of guanidine hydrochloride. Biochemistry. 1975 Jul 15;14(14):3069–3074. doi: 10.1021/bi00685a005. [DOI] [PubMed] [Google Scholar]
- Brown M. F., Schleich T. Resolution of independently titrating spectral components in the ultraviolet circular dichroism of subtilisin enzymes by matrix rank analysis. Biochim Biophys Acta. 1977 Nov 23;485(1):37–51. doi: 10.1016/0005-2744(77)90191-7. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Chau K. H. Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry. 1974 Jul 30;13(16):3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- Edelhoch H., Brand L., Wilchek M. Fluorescence studies with tryptophyl peptides. Biochemistry. 1967 Feb;6(2):547–559. doi: 10.1021/bi00854a024. [DOI] [PubMed] [Google Scholar]
- Genov N., Shopova M., Boteva R., Jori G., Ricchelli F. Chemical, photochemical and spectroscopic characterization of an alkaline proteinase from Bacillus subtilis variant DY. Biochem J. 1982 Nov 1;207(2):193–200. doi: 10.1042/bj2070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genov N. Spectrophotometric titration of tyrosyl residues in alkaline mesentericopeptidase. Int J Pept Protein Res. 1975;7(4):325–332. doi: 10.1111/j.1399-3011.1975.tb02447.x. [DOI] [PubMed] [Google Scholar]
- Gounaris A., Ottesen M. Some properties of succinylated subtilopeptidase. C R Trav Lab Carlsberg. 1965;35(3):37–62. [PubMed] [Google Scholar]
- Grossweiner L. I. Application of diffusion theory to photodynamic damage in large targets. Photochem Photobiol. 1977 Sep;26(3):309–311. doi: 10.1111/j.1751-1097.1977.tb07490.x. [DOI] [PubMed] [Google Scholar]
- Herskovits T. T., Fuchs H. H. Solvent perturbation of the tyrosyl and tryptophyl residues in subtilisin BPN'. Biochim Biophys Acta. 1972 May 18;263(3):468–476. doi: 10.1016/0005-2795(72)90028-1. [DOI] [PubMed] [Google Scholar]
- Markland F. S., Brown D. M., Smith E. L. Subtilisin Amylosacchariticus. I. Physicochemical characterization. J Biol Chem. 1972 Sep 10;247(17):5596–5601. [PubMed] [Google Scholar]
- Markland F. S. Phenolic hydroxyl ionization in two subtilisins. J Biol Chem. 1969 Feb 25;244(4):694–700. [PubMed] [Google Scholar]
- Stauffer C. E., Sullivan J. F. The effect of urea and guanidinium chloride on activity of subtilisin Carlsberg. Biochim Biophys Acta. 1971 Dec 28;251(3):407–412. doi: 10.1016/0005-2795(71)90129-2. [DOI] [PubMed] [Google Scholar]
- Tachibana A., Murachi T. Phenolic hydroxyl ionization in stem bromelain. Biochemistry. 1966 Aug;5(8):2756–2763. doi: 10.1021/bi00872a036. [DOI] [PubMed] [Google Scholar]
- Von Hippel P. H., Wong K. Y. On the conformational stability of globular proteins. The effects of various electrolytes and nonelectrolytes on the thermal ribonuclease transition. J Biol Chem. 1965 Oct;240(10):3909–3923. [PubMed] [Google Scholar]
- Wright C. S., Alden R. A., Kraut J. Structure of subtilisin BPN' at 2.5 angström resolution. Nature. 1969 Jan 18;221(5177):235–242. doi: 10.1038/221235a0. [DOI] [PubMed] [Google Scholar]


