Abstract
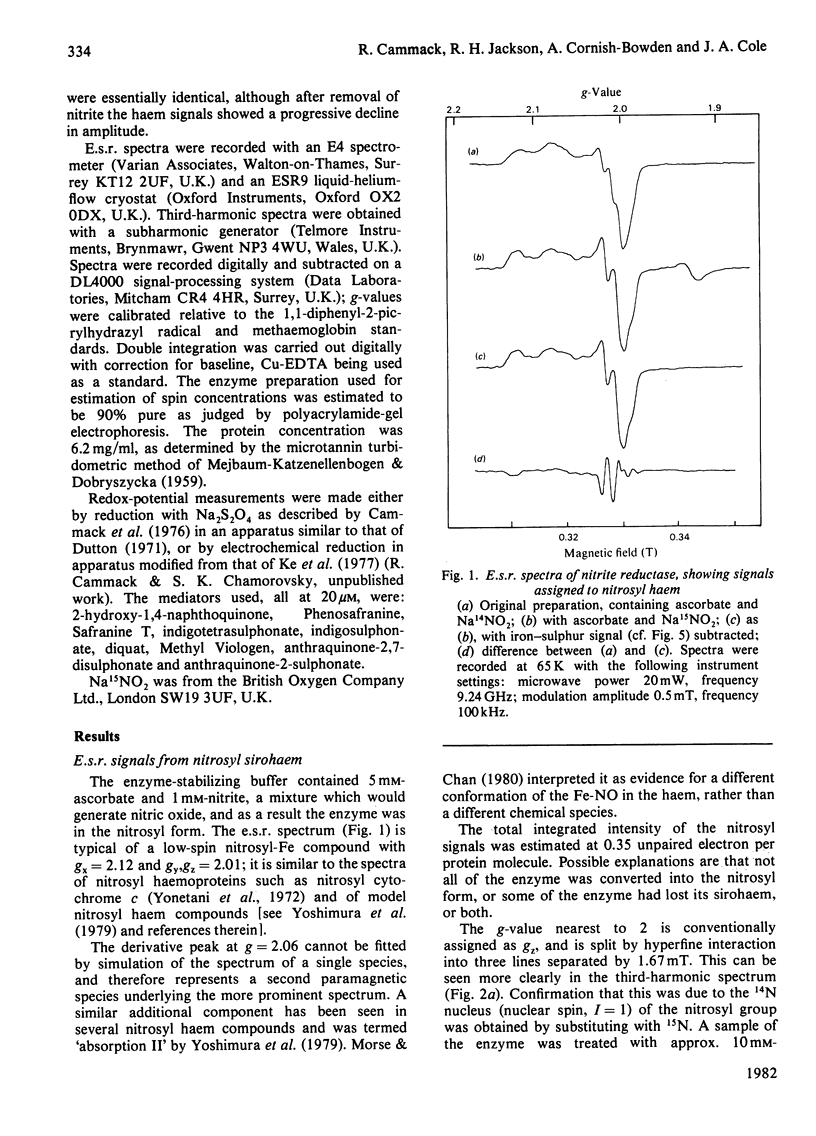
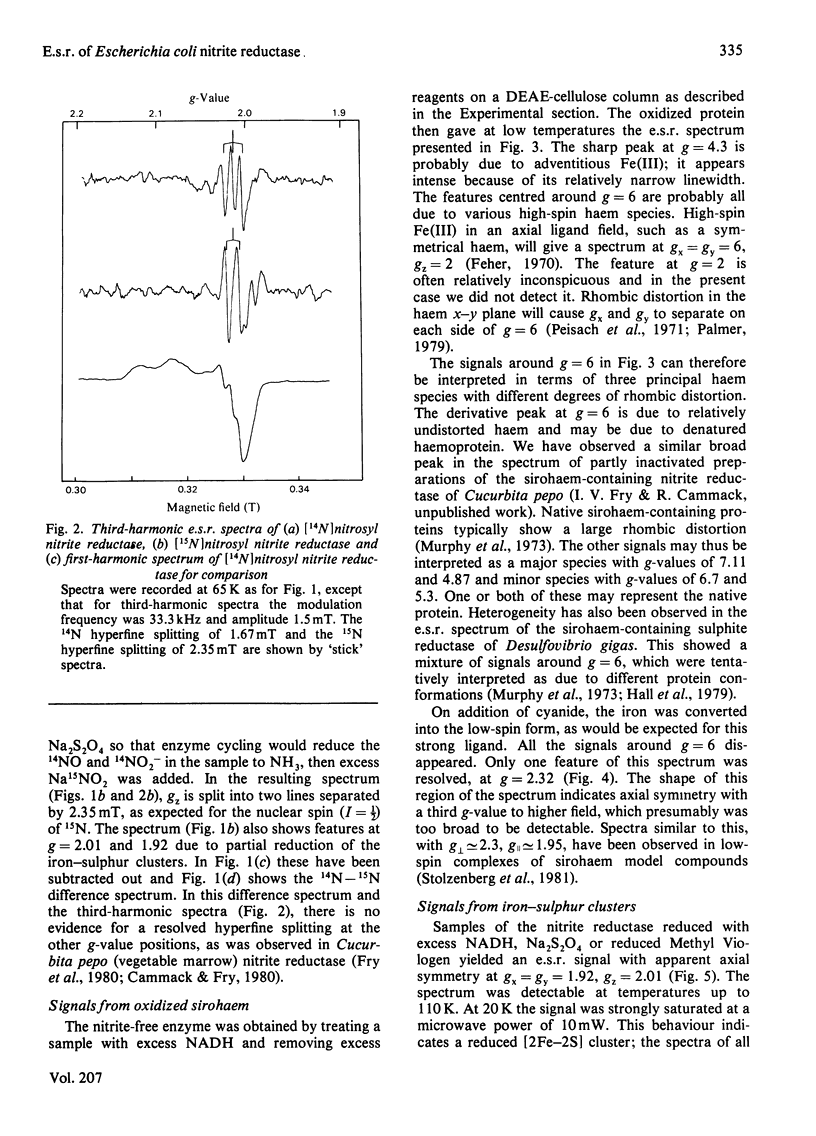
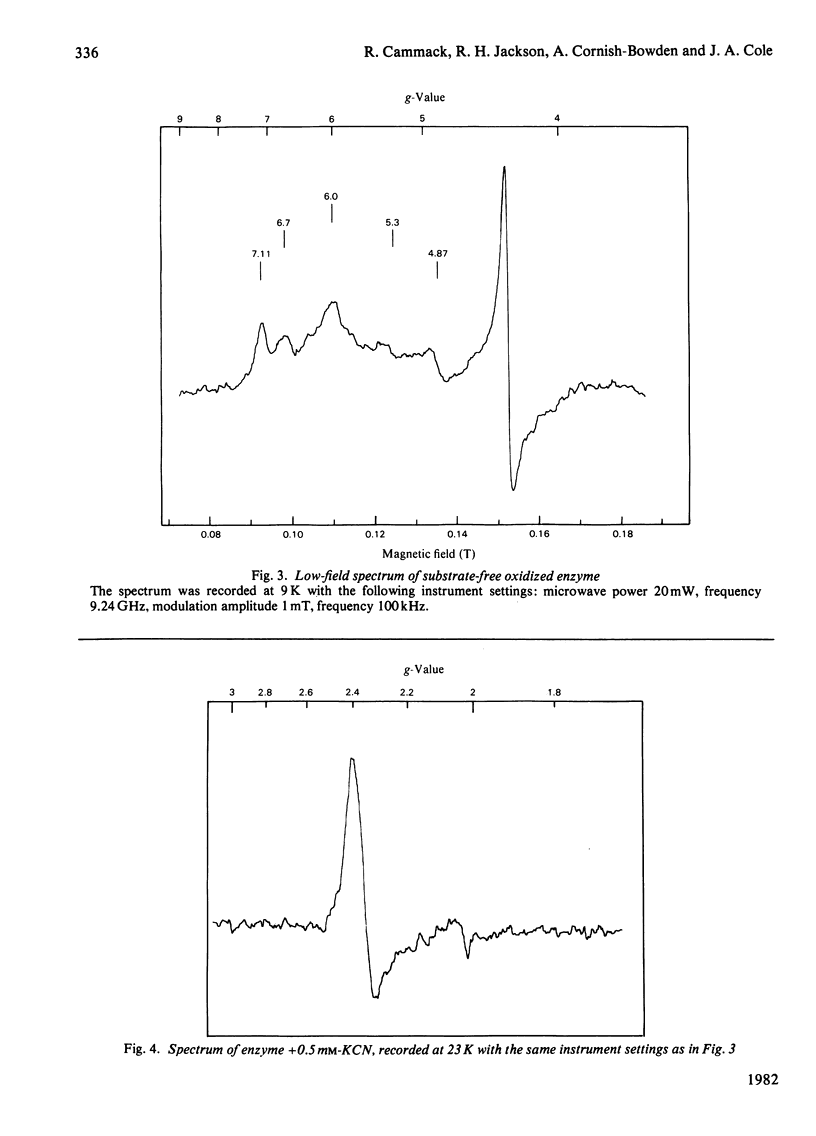
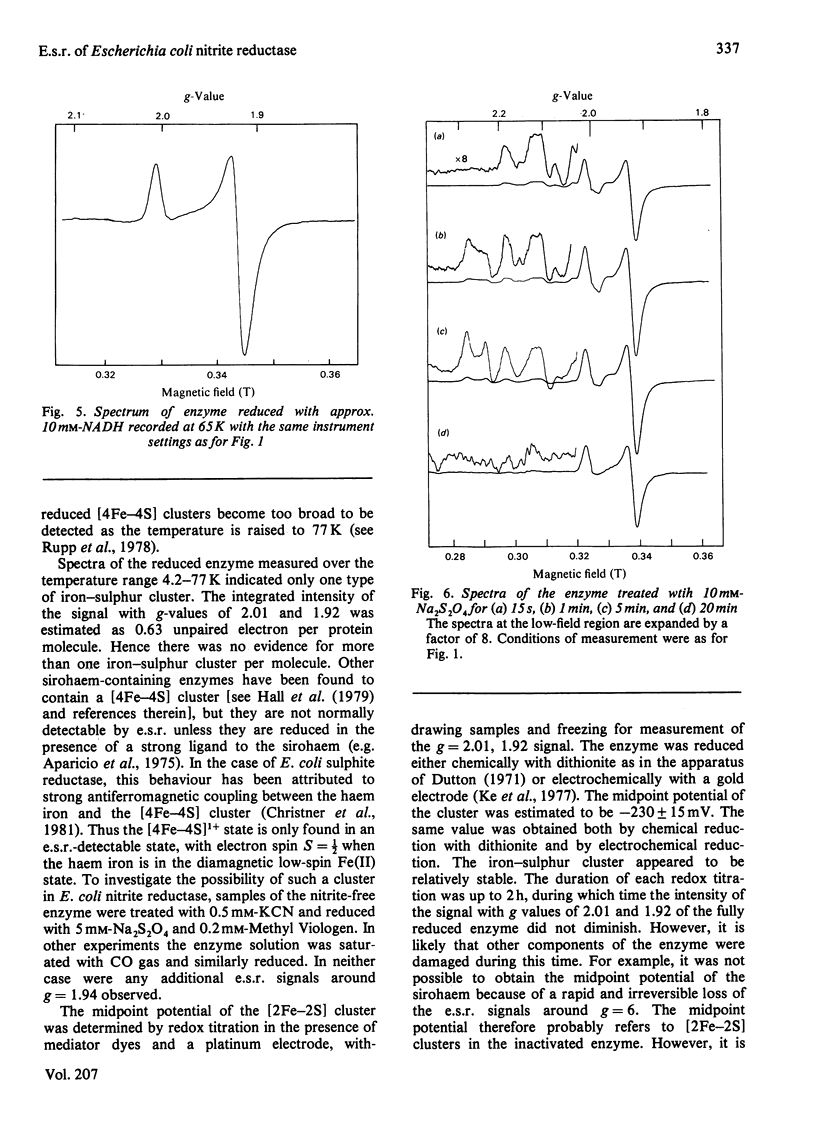
The NADH-dependent nitrite reductase of Escherichia coli, which contains sirohaem, flavin, non-haem iron and labile sulphide, was examined by low-temperature e.s.r. spectroscopy. The enzyme, stored in the presence of nitrite and ascorbate, gave the spectrum of a nitrosyl derivative, with hyperfine splitting due to the nitrosyl nitrogen. On removal of these reagents, a series of signals centred around g = 6 was observed, typical of high-spin ferric haem. Cyanide converted this into a low-spin form. On reduction of the enzyme with NADH, an axial spectrum at g = 1.92, 2.01 was observed. The temperature-dependence of this signal is indicative of a [2Fe-2S] iron-sulphur cluster. The midpoint potential of this cluster was estimated to be -230 +/- 15 mV by two independent methods. Reduction of the enzyme with dithionite yielded further signals, which are at present unidentified, at g = 2.1-2.28. No signals were observed that could be assigned to a [4Fe-4S] cluster, such as is found in other sulphite reductases and nitrite reductases that contain sirohaem.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aparicio P. J., Knaff D. B., Malkin R. The role of an iron-sulfur center and siroheme in spinach nitrite reductase. Arch Biochem Biophys. 1975 Jul;169(1):102–107. doi: 10.1016/0003-9861(75)90321-5. [DOI] [PubMed] [Google Scholar]
- Cammack R., Barber M. J., Bray R. C. Oxidation-reduction potentials of molybdenum, flavin and iron-sulphur centres in milk xanthine oxidase. Biochem J. 1976 Aug 1;157(2):469–478. doi: 10.1042/bj1570469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammack R., Fry I. V. Third-harmonic detection of electron-paramagnetic-resonance spectra: resolution of the hyperfine splitting in nitrosylated nitrite reductase from vegetable marrow (Cucurbita pepo). Biochem Soc Trans. 1980 Oct;8(5):642–642. doi: 10.1042/bst0080642. [DOI] [PubMed] [Google Scholar]
- Cammack R., Hucklesby D. P., Hewitt E. J. Electron-paramagnetic-resonance studies of the mechanism of leaf nitrite reductase. Signals from the iron-sulphur centre and haem under turnover conditions. Biochem J. 1978 Jun 1;171(3):519–526. doi: 10.1042/bj1710519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman K. J., Cornish-Bowden A., Cole J. A. Purification and properties of nitrite reductase from Escherichia coli K12. Biochem J. 1978 Nov 1;175(2):483–493. doi: 10.1042/bj1750483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton P. L. Oxidation-reduction potential dependence of the interaction of cytochromes, bacteriochlorophyll and carotenoids at 77 degrees K in chromatophores of Chromatium D and Rhodopseudomonas gelatinosa. Biochim Biophys Acta. 1971 Jan 12;226(1):63–80. doi: 10.1016/0005-2728(71)90178-2. [DOI] [PubMed] [Google Scholar]
- Fry I. V., Cammack R., Hucklesby D. P., Hewitt E. J. Stability of the nitrosyl-sirohaem complex of plant nitrite reductase, investigated by EPR spectroscopy. FEBS Lett. 1980 Mar 10;111(2):377–380. doi: 10.1016/0014-5793(80)80831-3. [DOI] [PubMed] [Google Scholar]
- Fujita T., Sato R. Studies on soluble cytochromes in Enterobacteriaceae. IV. Possible involvement of cytochrome c-552 in anaerobic nitrite metabolism. J Biochem. 1966 Dec;60(6):691–700. doi: 10.1093/oxfordjournals.jbchem.a128495. [DOI] [PubMed] [Google Scholar]
- Hall M. H., Prince R. H., Cammack R. EPR spectroscopy of the iron-sulphur cluster and sirohaem in the dissimilatory sulphite reductase (desulphoviridin) from Desulphovibrio gigas. Biochim Biophys Acta. 1979 Nov 23;581(1):27–33. doi: 10.1016/0005-2795(79)90217-4. [DOI] [PubMed] [Google Scholar]
- Jackson R. H., Cole J. A., Cornish-Bowden A. The steady state kinetics of the NADH-dependent nitrite reductase from Escherichia coli K12. The reduction of single-electron acceptors. Biochem J. 1982 May 1;203(2):505–510. doi: 10.1042/bj2030505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. H., Cole J. A., Cornish-Bowden A. The steady-state kinetics of the NADH-dependent nitrite reductase from Escherichia coli K 12. Nitrite and hydroxylamine reduction. Biochem J. 1981 Oct 1;199(1):171–178. doi: 10.1042/bj1990171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEMP J. D., ATKINSON D. E., EHRET A., LAZZARINI R. A. EVIDENCE FOR THE IDENTITY OF THE NICOTINAMIDE ADENINE DINUCLEOTIDE PHOSPHATE-SPECIFIC SULFITE AND NITRITE REDUCTASES OF ESCHERICHIA COLI. J Biol Chem. 1963 Oct;238:3466–3471. [PubMed] [Google Scholar]
- Lancaster J. R., Vega J. M., Kamin H., Orme-Johnson N. R., Orme-Johnson W. H., Krueger R. J., Siegel L. M. Identification of the iron-sulfur center of spinach ferredoxin-nitrite reductase as a tetranuclear center, and preliminary EPR studies of mechanism. J Biol Chem. 1979 Feb 25;254(4):1268–1272. [PubMed] [Google Scholar]
- Morse R. H., Chan S. I. Electron paramagnetic resonance studies of nitrosyl ferrous heme complexes. Determination of an equilibrium between two conformations. J Biol Chem. 1980 Aug 25;255(16):7876–7882. [PubMed] [Google Scholar]
- Murphy M. J., Siegel L. M., Kamin H., DerVartanian D. V., Lee J. P., LeGall J., Peck H. D., Jr An iron tetrahydroporphyrin prosthetic group common to both assimilatory and dissimilatory sulfite reductases. Biochem Biophys Res Commun. 1973 Sep 5;54(1):82–88. doi: 10.1016/0006-291x(73)90891-7. [DOI] [PubMed] [Google Scholar]
- Peisach J., Blumberg W. E., Ogawa S., Rachmilewitz E. A., Oltzik R. The effects of protein conformation on the heme symmetry in high spin ferric heme proteins as studied by electron paramagnetic resonance. J Biol Chem. 1971 May 25;246(10):3342–3355. [PubMed] [Google Scholar]
- Rupp H., Rao K. K., Hall D. O., Cammack R. Electron spin relaxation of iron-sulphur proteins studied by microwave power saturation. Biochim Biophys Acta. 1978 Dec 20;537(2):255–260. doi: 10.1016/0005-2795(78)90509-3. [DOI] [PubMed] [Google Scholar]
- Yonetani T., Yamamoto H., Erman J. E., Leigh J. S., Jr, Reed G. H. Electromagnetic properties of hemoproteins. V. Optical and electron paramagnetic resonance characteristics of nitric oxide derivatives of metalloporphyrin-apohemoprotein complexes. J Biol Chem. 1972 Apr 25;247(8):2447–2455. [PubMed] [Google Scholar]
- Yoshimura T., Ozaki T., Shintani Y., Watanabe H. Electron paramagnetic resonance of nitrosylprotoheme dimethyl ester complexes with imidazole derivatives as model systems for nitrosylhemoproteins. Arch Biochem Biophys. 1979 Apr 1;193(2):301–313. doi: 10.1016/0003-9861(79)90035-3. [DOI] [PubMed] [Google Scholar]


