Abstract
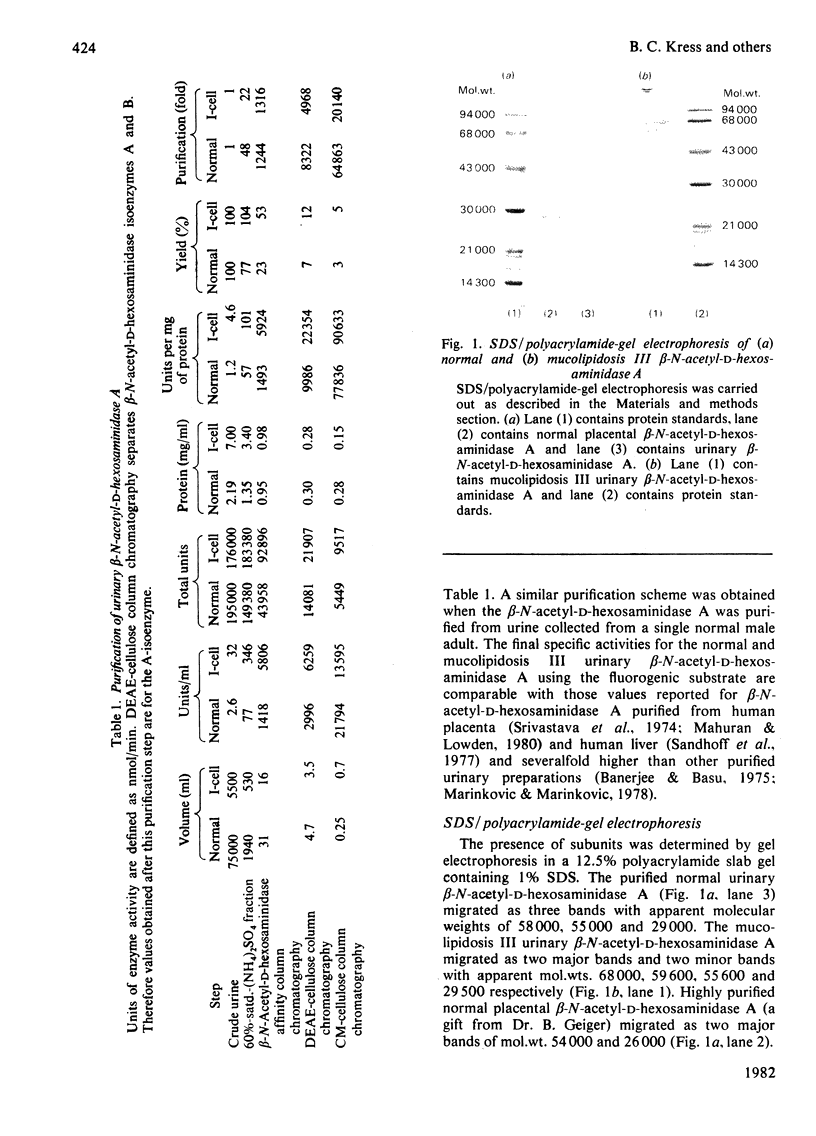
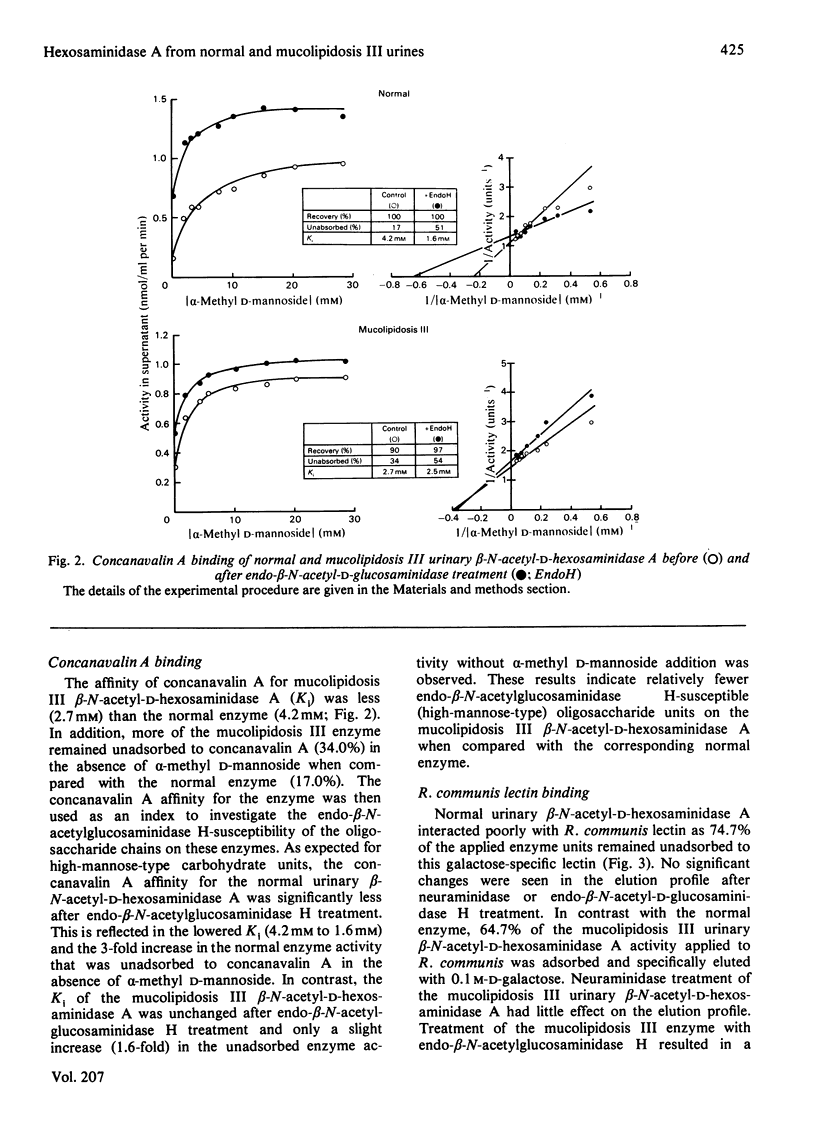
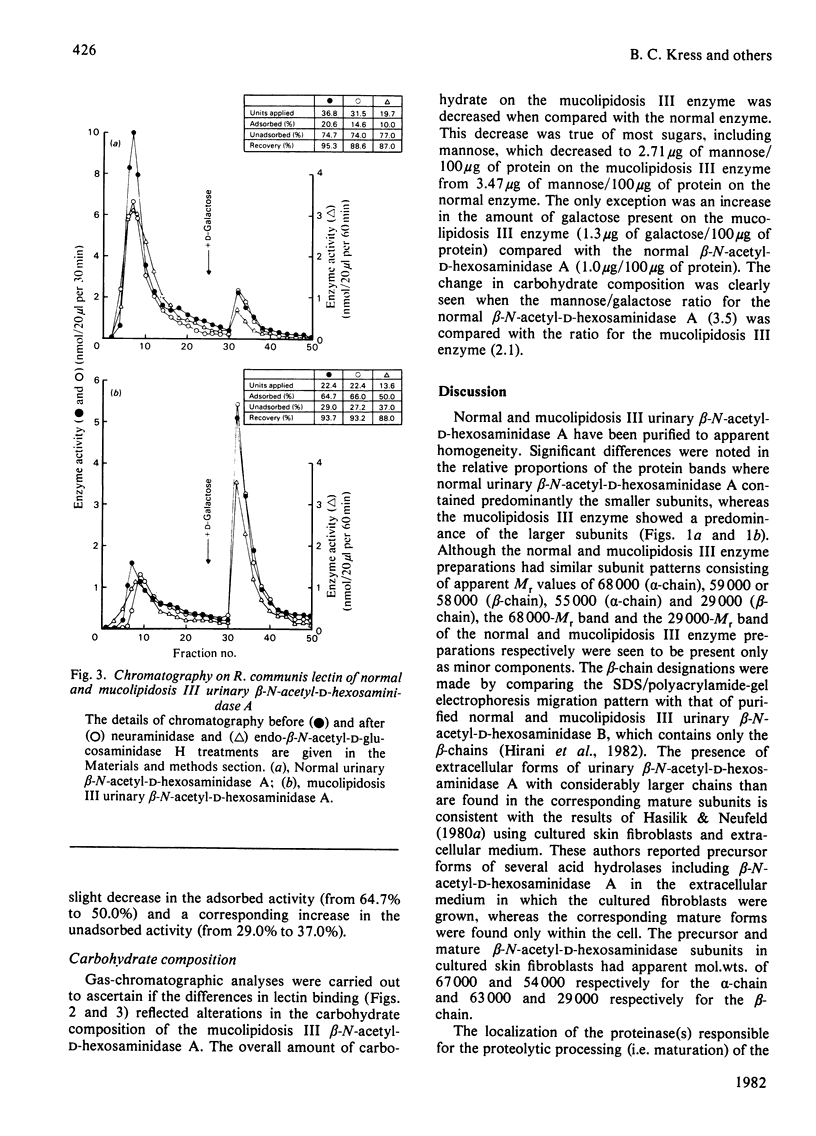
Mucolipidosis III acid hydrolases possess an altered carbohydrate recognition marker needed for their lysosomal localization. As a result of this alteration, a portion of these enzymes is secreted from the cell to the extracellular spaces. The structural changes that may have occurred to one of these secreted enzymes, β-N-acetyl-d-hexosaminidase A (EC 3.2.1.52) were investigated. Normal and mucolipidosis III urinary β-N-acetyl-d-hexosaminidase A were purified to apparent homogeneity by using affinity [Sepharose-2-acetamido-N-(ε-aminocaproyl)-2-deoxy-β- d-glucopyranosylamine] and ion-exchange (DEAE- and CM-cellulose) chromatography. Sodium dodecyl sulphate/polyacrylamide-slab-gel electrophoresis showed that both enzymes had similar subunit patterns consisting of apparent mol.wts. of 68000, 60000–58000, 55000 and 29000. Differences, however, were noted in the relative proportions of the protein bands where the normal urinary β-N-acetyl-d-hexosaminidase A contained predominantly the smaller subunits, whereas the mucolipidosis III enzyme had a predominance of the larger subunits. The binding of mucolipidosis III β-N-acetyl-d-hexosaminidase A to Ricinus communis lectin and concanavalin A with and without endo-β-N-acetyl-d-glucosaminidase H treatment indicated that the mutation leads to a modification of a portion of the normally occurring high-mannose-type oligosaccharide units to the complex-type. This was further supported by carbohydrate compositional analysis, which revealed a mannose/galactose ratio of 2.1 for the mucolipidosis III β-N-acetyl-d-hexosaminidase A compared with a ratio of 3.5 for the normal enzyme. Our results indicate that as a result of their inability to be properly localized to the lysosome the majority of the mucolipidosis III lysosomal hydrolase high-mannose oligosaccharide units are further processed to the complex-type before secretion of predominantly higher-molecular-weight subunits from the cell.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee D. K., Basu D. Purification of normal human urinary N-acetyl-beta-hexosaminidase A by affinity chromatography. Biochem J. 1975 Jan;145(1):113–118. doi: 10.1042/bj1450113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Tandt W. R., Lassila E., Philippart M. Leroy's l-cell disease: markedly increased activity of plasma acid hydrolases. J Lab Clin Med. 1974 Mar;83(3):403–408. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Freeze H., Geiger B., Miller A. L. Carbohydrate composition of human placental N-acetylhexosaminidase A and B. Biochem J. 1979 Feb 1;177(2):749–752. doi: 10.1042/bj1770749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch A., Neufeld E. F. Limited proteolysis of the beta-hexosaminidase precursor in a cell-free system. J Biol Chem. 1981 Aug 10;256(15):8242–8246. [PubMed] [Google Scholar]
- Hasilik A., Klein U., Waheed A., Strecker G., von Figura K. Phosphorylated oligosaccharides in lysosomal enzymes: identification of alpha-N-acetylglucosamine(1)phospho(6)mannose diester groups. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7074–7078. doi: 10.1073/pnas.77.12.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Phosphorylation of mannose residues. J Biol Chem. 1980 May 25;255(10):4946–4950. [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Hasilik A., Von Figura K. Oligosaccharides in lysosomal enzymes. Distribution of high-mannose and complex oligosaccharides in cathepsin D and beta-hexosaminidase. Eur J Biochem. 1981 Dec;121(1):125–129. doi: 10.1111/j.1432-1033.1981.tb06440.x. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Waheed A., von Figura K. Enzymatic phosphorylation of lysosomal enzymes in the presence of UDP-N-acetylglucosamine. Absence of the activity in I-cell fibroblasts. Biochem Biophys Res Commun. 1981 Feb 12;98(3):761–767. doi: 10.1016/0006-291x(81)91177-3. [DOI] [PubMed] [Google Scholar]
- Herd J. K., Dvorak A. D., Wiltse H. E., Eisen J. D., Kress B. C., Miller A. L. Mucolipidosis type III. Multiple elevated serum and urine enzyme activities. Am J Dis Child. 1978 Dec;132(12):1181–1186. doi: 10.1001/archpedi.1978.02120370029007. [DOI] [PubMed] [Google Scholar]
- Hickman S., Neufeld E. F. A hypothesis for I-cell disease: defective hydrolases that do not enter lysosomes. Biochem Biophys Res Commun. 1972 Nov 15;49(4):992–999. doi: 10.1016/0006-291x(72)90310-5. [DOI] [PubMed] [Google Scholar]
- Hirani S., Little L., Miller A. L. A study of highly purified mucolipidosis III urinary N-acetyl-beta-D-hexosaminidase B. Biochem J. 1982 May 15;204(2):557–563. doi: 10.1042/bj2040557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress B. C., Freeze H. H., Herd J. K., Alhadeff J. A., Miller A. L. Purification and characterization of I-cell disease alpha-L-fucosidase. J Biol Chem. 1980 Feb 10;255(3):955–961. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leroy J. G., Ho M. W., MacBrinn M. C., Zielke K., Jacob J., O'Brien J. S. I-cell disease: biochemical studies. Pediatr Res. 1972 Oct;6(10):752–757. doi: 10.1203/00006450-197210000-00002. [DOI] [PubMed] [Google Scholar]
- Mahuran D., Lowden J. A. The subunit and polypeptide structure of hexosaminidases from human placenta. Can J Biochem. 1980 Apr;58(4):287–294. doi: 10.1139/o80-038. [DOI] [PubMed] [Google Scholar]
- Marinkovic D. V., Marinkovic J. N. Purification and properties of N-acetyl-beta-hexosaminidases from human urine. Biochem Med. 1978 Dec;20(3):422–433. doi: 10.1016/0006-2944(78)90092-3. [DOI] [PubMed] [Google Scholar]
- Miller A. L., Kress B. C., Lewis L., Stein R., Kinnon C. Effect of tunicamycin and cycloheximide on the secretion of acid hydrolases from I-cell cultured fibroblasts. Biochem J. 1980 Mar 15;186(3):971–975. doi: 10.1042/bj1860971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitman M. L., Varki A., Kornfeld S. Fibroblasts from patients with I-cell disease and pseudo-Hurler polydystrophy are deficient in uridine 5'-diphosphate-N-acetylglucosamine: glycoprotein N-acetylglucosaminylphosphotransferase activity. J Clin Invest. 1981 May;67(5):1574–1579. doi: 10.1172/JCI110189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey P. G., Neufeld E. F. Defective phosphorylation and processing of beta-hexosaminidase by intact cultured fibroblasts from patients with mucolipidosis III. Arch Biochem Biophys. 1982 Jan;213(1):251–257. doi: 10.1016/0003-9861(82)90459-3. [DOI] [PubMed] [Google Scholar]
- Sandhoff K., Conzelmann E., Nehrkorn H. Specificity of human liver hexosaminidases A and B against glycosphingolipids GM2 and GA2. Purification of the enzymes by affinity chromatography employing specific elution. Hoppe Seylers Z Physiol Chem. 1977 Jul;358(7):779–787. doi: 10.1515/bchm2.1977.358.2.779. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Awasthi Y. C., Yoshida A., Beutler E. Studies on human beta-D-N-acetylhexosaminidases. I. Purification and properties. J Biol Chem. 1974 Apr 10;249(7):2043–2048. [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. Biosynthetic intermediates of beta-glucuronidase contain high mannose oligosaccharides with blocked phosphate residues. J Biol Chem. 1980 Jul 25;255(14):6633–6639. [PubMed] [Google Scholar]
- Varki A., Kornfeld S. Identification of a rat liver alpha-N-acetylglucosaminyl phosphodiesterase capable of removing "blocking" alpha-N-acetylglucosamine residues from phosphorylated high mannose oligosaccharides of lysosomal enzymes. J Biol Chem. 1980 Sep 25;255(18):8398–8401. [PubMed] [Google Scholar]
- Vladutiu G. D., Rattazzi M. C. The effect of monensin on beta-hexosaminidase transport in normal and I-cell fibroblasts. Biochem J. 1980 Dec 15;192(3):813–820. doi: 10.1042/bj1920813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger D. A., Sattler M., Clark C., Wharton C. I-cell disease: activities of lysosomal enzymes toward natural and synthetic substrates. Life Sci. 1976 Aug 1;19(3):413–420. doi: 10.1016/0024-3205(76)90047-3. [DOI] [PubMed] [Google Scholar]
- von Figura K., Klein U. Isolation and characterization of phosphorylated oligosaccharides from alpha-N-acetylglucosaminidase that are recognized by cell-surface receptors. Eur J Biochem. 1979 Mar;94(2):347–354. doi: 10.1111/j.1432-1033.1979.tb12900.x. [DOI] [PubMed] [Google Scholar]



