Abstract
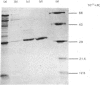
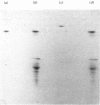
A protein with pore-forming activity has been isolated from the outer membrane of rat liver mitochondria. The purification involves sucrose gradient centrifugation, differential centrifugation in the presence of Triton X-100, and DEAE-Sepharose and CM-Sepharose chromatography. The yield of the purified protein was approx. 2% of the total outer membrane proteins. The protein, when inserted into soya bean phospholipid vesicles, increases the [3H]sucrose permeability of the vesicles but had no effect on the permeability of high-molecular-weight [14C]dextran (Mr 70 000). The protein is very active, since as little as 3-4 micrograms of protein per mg of phospholipid is required for the complete release of [3H]sucrose from the vesicles. Sucrose diffusion channels could not be reconstituted with other membrane proteins such as rat liver cytochrome oxidase or cytochrome b5. Purified pore protein revealed a single band of apparent Mr 30000 when resolved by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. This polypeptide could be further resolved by isoelectric focusing into a major (pI7.9) and two relatively minor (pI7.6 and 7.2) components. Proteolytic mapping with V8 proteinase from Staphylococcus aureus suggests that these probably represent a single component showing charge heterogeneity. The reason for the charge heterogeneity is not known. The amino acid composition of the protein revealed 47.8% polar amino acids with a relatively high lysine content.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brierley G., O'Brien R. L. Compartmentation of heart mitochondria. II. Mitochondrial adenine nucleotides and the action of atractyloside. J Biol Chem. 1965 Nov;240(11):4532–4539. [PubMed] [Google Scholar]
- Capaldi R. A., Vanderkooi G. The low polarity of many membrane proteins. Proc Natl Acad Sci U S A. 1972 Apr;69(4):930–932. doi: 10.1073/pnas.69.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Krämer C., Schmidmayr W., Chen-Schmeisser U., Henning U. Primary structure of major outer-membrane protein I (ompF protein, porin) of Escherichia coli B/r. Biochem J. 1982 Apr 1;203(1):33–43. doi: 10.1042/bj2030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Colombini M. A candidate for the permeability pathway of the outer mitochondrial membrane. Nature. 1979 Jun 14;279(5714):643–645. doi: 10.1038/279643a0. [DOI] [PubMed] [Google Scholar]
- Colombini M. Structure and mode of action of a voltage dependent anion-selective channel (VDAC) located in the outer mitochondrial membrane. Ann N Y Acad Sci. 1980;341:552–563. doi: 10.1111/j.1749-6632.1980.tb47198.x. [DOI] [PubMed] [Google Scholar]
- Elhammer A., Dallner G., Omura T. Glycosylation of rat liver cytochrome b5 on the ribosomal level. Biochem Biophys Res Commun. 1978 Oct 16;84(3):572–580. doi: 10.1016/0006-291x(78)90744-1. [DOI] [PubMed] [Google Scholar]
- Freitag H., Neupert W., Benz R. Purification and characterisation of a pore protein of the outer mitochondrial membrane from Neurospora crassa. Eur J Biochem. 1982 Apr;123(3):629–636. doi: 10.1111/j.1432-1033.1982.tb06578.x. [DOI] [PubMed] [Google Scholar]
- Gellerfors P., Lindén M. Biogenesis of the outer mitochondrial membrane in isolated rat hepatocytes. FEBS Lett. 1981 May 5;127(1):91–93. doi: 10.1016/0014-5793(81)80348-1. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Decad G. M., Nikaido H. Identification of the protein producing transmembrane diffusion pores in the outer membrane of Pseudomonas aeruginosa PA01. Biochim Biophys Acta. 1979 Jul 5;554(2):323–331. doi: 10.1016/0005-2736(79)90373-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luckey M., Nikaido H. Diffusion of solutes through channels produced by phage lambda receptor protein of Escherichia coli: inhibition by higher oligosaccharides of maltose series. Biochem Biophys Res Commun. 1980 Mar 13;93(1):166–171. doi: 10.1016/s0006-291x(80)80261-0. [DOI] [PubMed] [Google Scholar]
- Mannella C. A., Bonner W. D., Jr X-ray diffraction from oriented outer mitochondrial membranes. Detection of in-plane subunit structure. Biochim Biophys Acta. 1975 Dec 1;413(2):226–233. doi: 10.1016/0005-2736(75)90106-6. [DOI] [PubMed] [Google Scholar]
- Nakae T. Identification of the outer membrane protein of E. coli that produces transmembrane channels in reconstituted vesicle membranes. Biochem Biophys Res Commun. 1976 Aug 9;71(3):877–884. doi: 10.1016/0006-291x(76)90913-x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Parsons D. F., Williams G. R., Chance B. Characteristics of isolated and purified preparations of the outer and inner membranes of mitochondria. Ann N Y Acad Sci. 1966 Jul 14;137(2):643–666. doi: 10.1111/j.1749-6632.1966.tb50188.x. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Pfaff E., Klingenberg M., Heldt H. W. Unspecific permeation and specific exchange of adenine nucleotides in liver mitochondria. Biochim Biophys Acta. 1965 Jun 15;104(1):312–315. doi: 10.1016/0304-4165(65)90258-8. [DOI] [PubMed] [Google Scholar]
- Roos N., Benz R., Brdiczka D. Identification and characterization of the pore-forming protein in the outer membrane of rat liver mitochondria. Biochim Biophys Acta. 1982 Apr 7;686(2):204–214. doi: 10.1016/0005-2736(82)90114-6. [DOI] [PubMed] [Google Scholar]
- WERKHEISER W. C., BARTLEY W. The study of steady-state concentrations of internal solutes of mitochondria by rapid centrifugal transfer to a fixation medium. Biochem J. 1957 May;66(1):79–91. doi: 10.1042/bj0660079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielburski A., Kuźela S., Nelson B. D. Studies on the assembly of cytochrome oxidase in isolated rat hepatocytes. Biochem J. 1982 Apr 15;204(1):239–245. doi: 10.1042/bj2040239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtczak L., Zaluska H. On the impermeability of the outer mitochondrial membrane to cytochrome c. I. Studies on whole mitochondria. Biochim Biophys Acta. 1969 Oct 14;193(1):64–72. doi: 10.1016/0005-2736(69)90059-5. [DOI] [PubMed] [Google Scholar]
- Zalman L. S., Nikaido H., Kagawa Y. Mitochondrial outer membrane contains a protein producing nonspecific diffusion channels. J Biol Chem. 1980 Mar 10;255(5):1771–1774. [PubMed] [Google Scholar]