Abstract
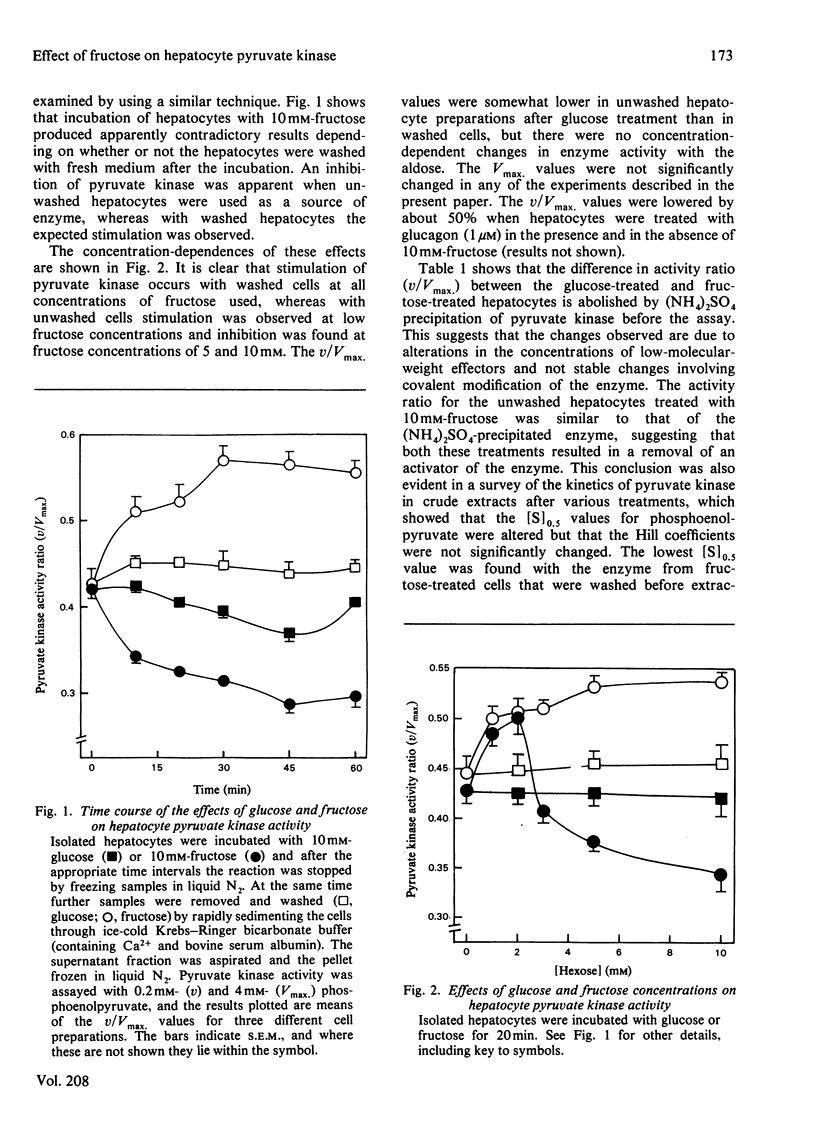
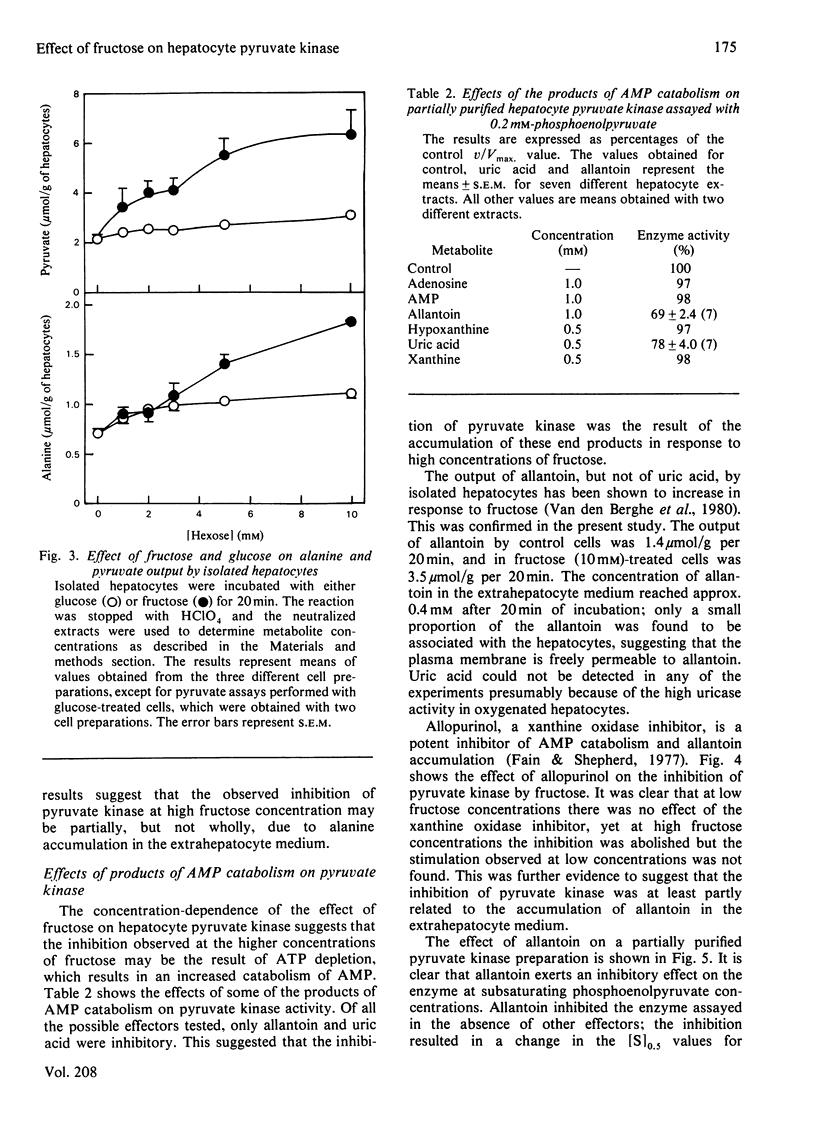
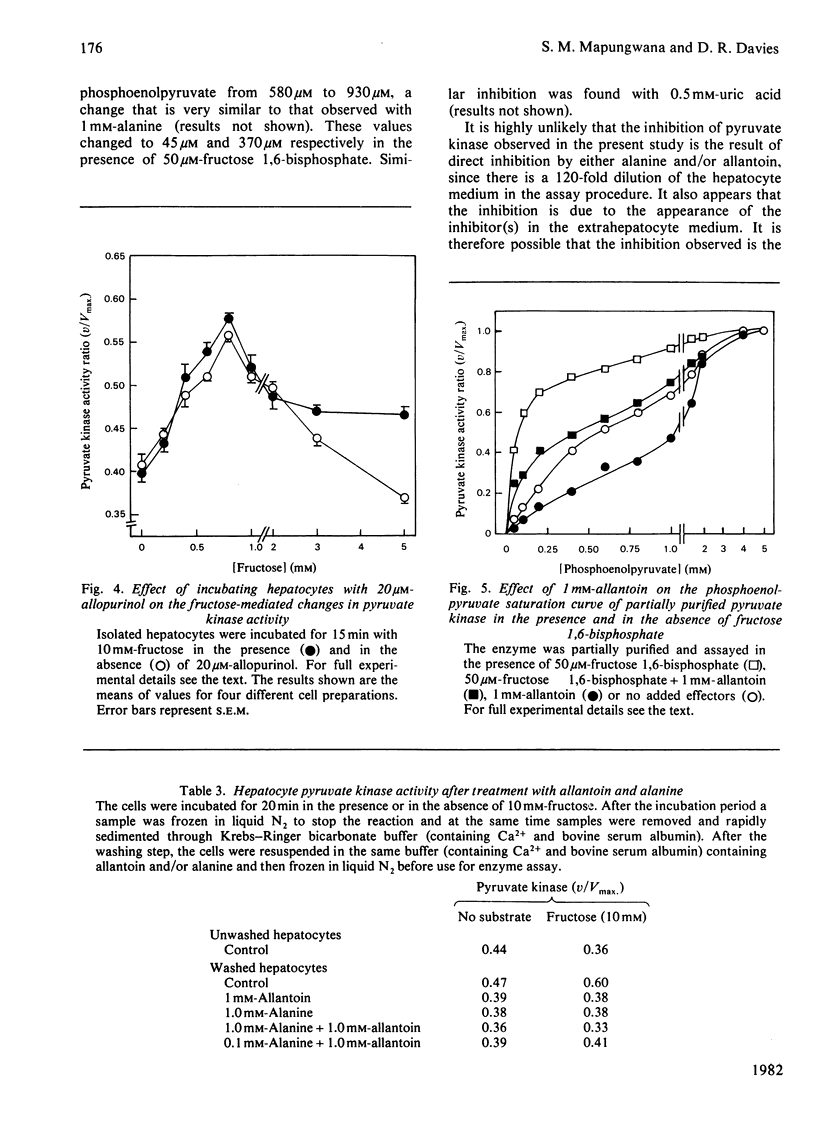
1. Incubation of isolated hepatocytes with fructose at concentrations above 3 mM resulted in an apparent inhibition of pyruvate kinase assayed in crude extracts at sub-optimal phosphoenolpyruvate concentrations. 2. Fructose at concentrations below 3 mM caused an activation of the enzyme. 3. Increases in the hepatocyte contents of the positive effectors fructose 1.6-bisphosphate and fructose 1-phosphate were found at all concentrations of fructose up to 10mM. 4. Removal of the extrahepatocyte medium from the hepatocytes by washing resulted in an activation of the enzyme at all concentrations of fructose examined. 5. Inhibitors of the enzyme were shown to accumulate in the hepatocytes despite the depletion of ATP (a known negative effector) caused by higher concentrations of fructose. Indeed the inhibition of pyruvate kinase appeared to be correlated to the depletion of ATP. 6. Alanine (a known inhibitor) was shown to accumulate in hepatocytes as a consequence of incubation with fructose. 7. Allantoin and uric acid were shown to be inhibitors of a partially purified pyruvate kinase preparation assayed both in the presence and in the absence of fructose 1.6-bisphosphate. 8. Allantoin, but not uric acid, accumulated in the extrahepatocyte medium as a result of incubation of the cells with 10 mM-fructose.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear P. J., Holloway P. A., Alberti K. G. Factors regulating amino acid release from extrasplanchnic tissues in the rat. Interactions of alanine and glutamine. Biochem J. 1975 Sep;150(3):379–387. doi: 10.1042/bj1500379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus T. H., El-Maghrabi M. R., Pilkis S. J. Modulation of the phosphorylation state of rat liver pyruvate kinase by allosteric effectors and insulin. J Biol Chem. 1979 Aug 25;254(16):7855–7864. [PubMed] [Google Scholar]
- Eggleston L. V., Woods H. F. Activation of liver pyruvate kinase by fructose-1-phosphate. FEBS Lett. 1970 Jan 15;6(1):43–45. doi: 10.1016/0014-5793(70)80038-2. [DOI] [PubMed] [Google Scholar]
- Fain J. N., Shepherd R. E. Adenosine, cyclic AMP metabolism, and glycogenolysis in rat liver cells. J Biol Chem. 1977 Nov 25;252(22):8066–8070. [PubMed] [Google Scholar]
- Feliú J. E., Hue L., Hers H. G. Hormonal control of pyruvate kinase activity and of gluconeogenesis in isolated hepatocytes. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2762–2766. doi: 10.1073/pnas.73.8.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOPPER S., SEGAL H. L. Kinetic studies of rat liver glutamicalanine transaminase. J Biol Chem. 1962 Oct;237:3189–3195. [PubMed] [Google Scholar]
- Imamura K., Taniuchi K., Tanaka T. Multimolecular forms of pyruvate kinase. II. Purification of M 2 -type pyruvate kinase from Yoshida ascites hepatoma 130 cells and comparative studies on the enzymological and immunological properties of the three types of pyruvate kinases, L, M 1 , and M 2 . J Biochem. 1972 Oct;72(4):1001–1015. doi: 10.1093/oxfordjournals.jbchem.a129962. [DOI] [PubMed] [Google Scholar]
- Irving M. G., Williams J. F. Studies on the interaction between rabbit liver pyruvate kinase and its allosteric effector fructose 1,6-diphosphate. Biochem J. 1973 Feb;131(2):303–313. doi: 10.1042/bj1310303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John R. A., Charteris A. The reaction of amino-oxyacetate with pyridoxal phosphate-dependent enzymes. Biochem J. 1978 Jun 1;171(3):771–779. doi: 10.1042/bj1710771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente P., Marco R., Sols A. Regulation of liver pyruvate kinase and the phosphoenolpyruvate crossroads. Eur J Biochem. 1970 Mar 1;13(1):45–54. doi: 10.1111/j.1432-1033.1970.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Mäenpä P. H., Raivio K. O., Kekomäki M. P. Liver adenine nucleotides: fructose-induced depletion and its effect on protein synthesis. Science. 1968 Sep 20;161(3847):1253–1254. doi: 10.1126/science.161.3847.1253. [DOI] [PubMed] [Google Scholar]
- Van den Berghe G., Bontemps F., Hers H. G. Purine catabolism in isolated rat hepatocytes. Influence of coformycin. Biochem J. 1980 Jun 15;188(3):913–920. doi: 10.1042/bj1880913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berghe G. Metabolic effects of fructose in the liver. Curr Top Cell Regul. 1978;13:97–135. doi: 10.1016/b978-0-12-152813-3.50008-2. [DOI] [PubMed] [Google Scholar]
- Vogels G. D., Van der Drift C. Differential analyses of glyoxylate derivatives. Anal Biochem. 1970 Jan;33(1):143–157. doi: 10.1016/0003-2697(70)90448-3. [DOI] [PubMed] [Google Scholar]
- Wagle S. R., Ingebretsen W. R., Jr Isolation, purification, and metabolic characteristics of rat liver hepatocytes. Methods Enzymol. 1975;35:579–594. doi: 10.1016/0076-6879(75)35186-0. [DOI] [PubMed] [Google Scholar]
- Woods H. F., Eggleston L. V., Krebs H. A. The cause of hepatic accumulation of fructose 1-phosphate on fructose loading. Biochem J. 1970 Sep;119(3):501–510. doi: 10.1042/bj1190501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berkel T. J., Kruijt J. K., Koster J. F. Hormone-induced changes in pyruvate kinase activity in isolated hepatocytes. II. Relation to the hormonal regulation of gluconeogenesis. Biochim Biophys Acta. 1977 Dec 22;500(2):267–276. doi: 10.1016/0304-4165(77)90019-8. [DOI] [PubMed] [Google Scholar]
- van Berkel T. J., Kruijt J. K., Koster J. F. Hormone-induced changes in pyruvate kinase. Effects of glucagon and starvation. Eur J Biochem. 1977 Dec;81(3):423–432. doi: 10.1111/j.1432-1033.1977.tb11967.x. [DOI] [PubMed] [Google Scholar]
- van Berkel T. J., Kruijt J. K., Koster J. F., Hülsmann W. C. Cyclic nucleotide-, pyruvate- and hormone-induced changes in pyruvate kinase activity in isolated rat hepatocytes. Biochem Biophys Res Commun. 1976 Oct 4;72(3):917–925. doi: 10.1016/s0006-291x(76)80219-7. [DOI] [PubMed] [Google Scholar]
- van den Berghe G., Bronfman M., Vanneste R., Hers H. G. The mechanism of adenosine triphosphate depletion in the liver after a load of fructose. A kinetic study of liver adenylate deaminase. Biochem J. 1977 Mar 15;162(3):601–609. doi: 10.1042/bj1620601. [DOI] [PMC free article] [PubMed] [Google Scholar]


