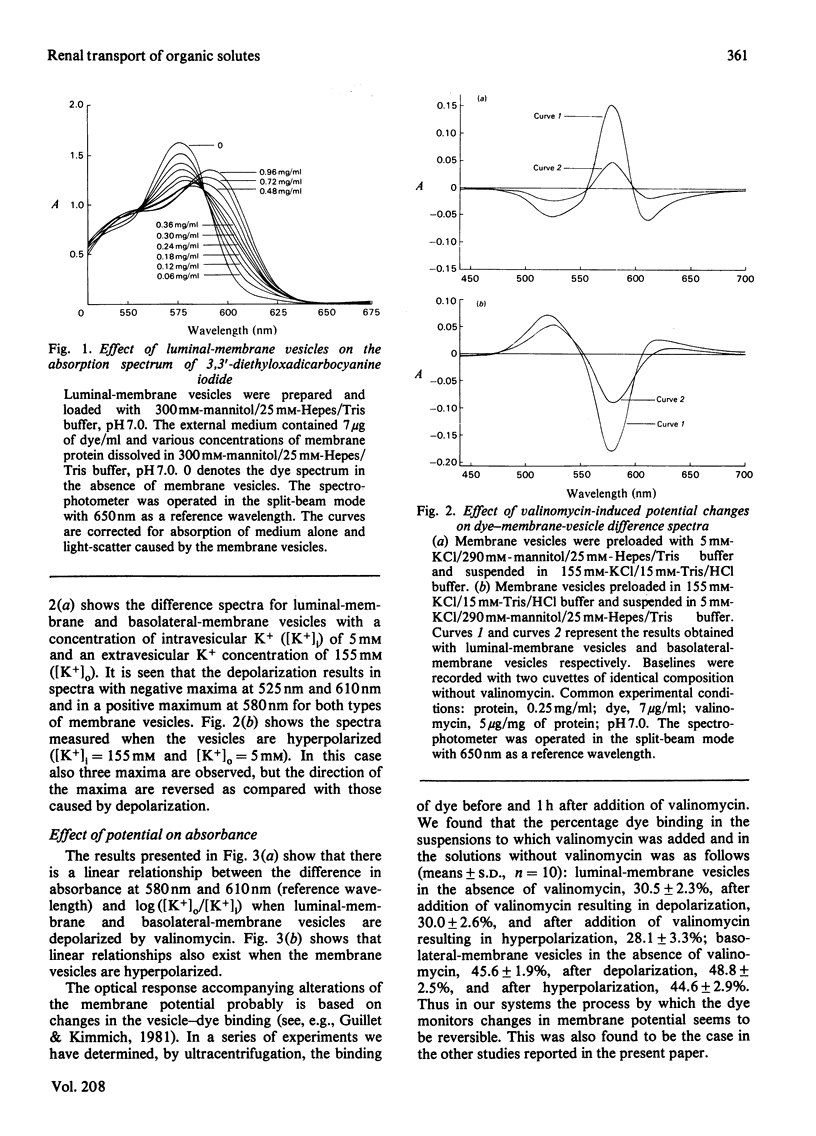
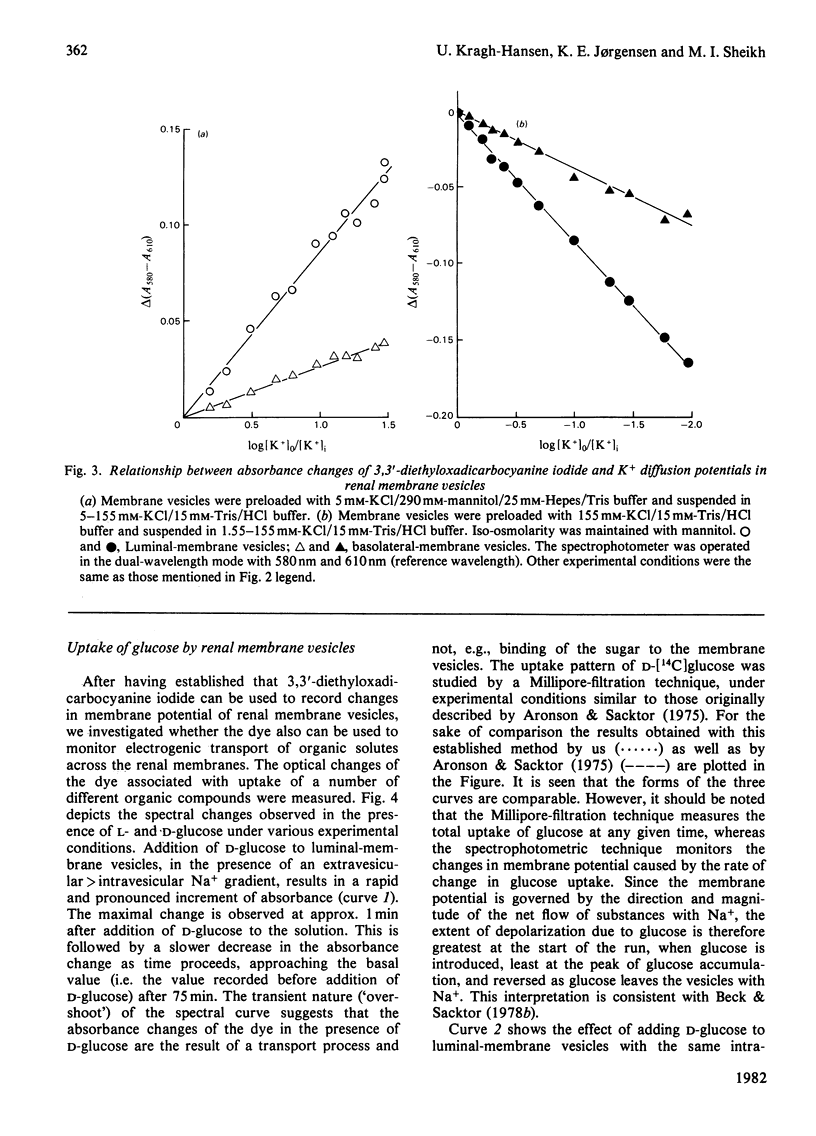
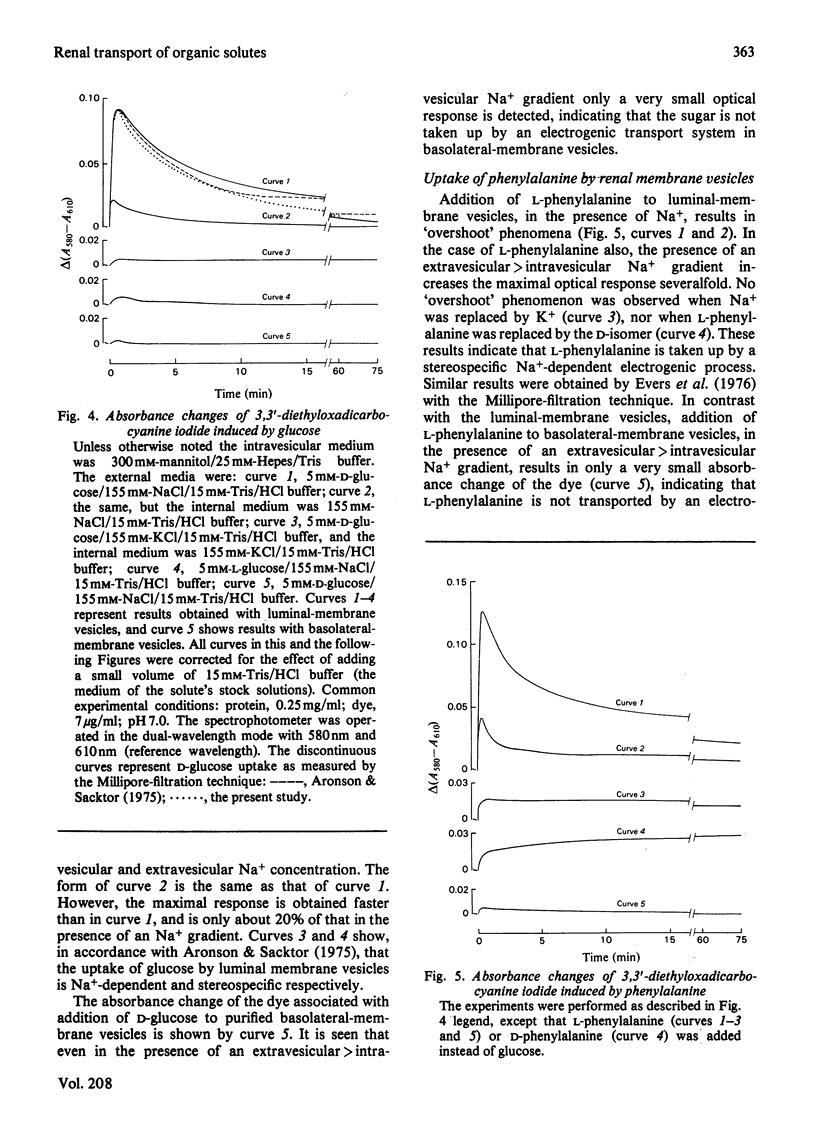
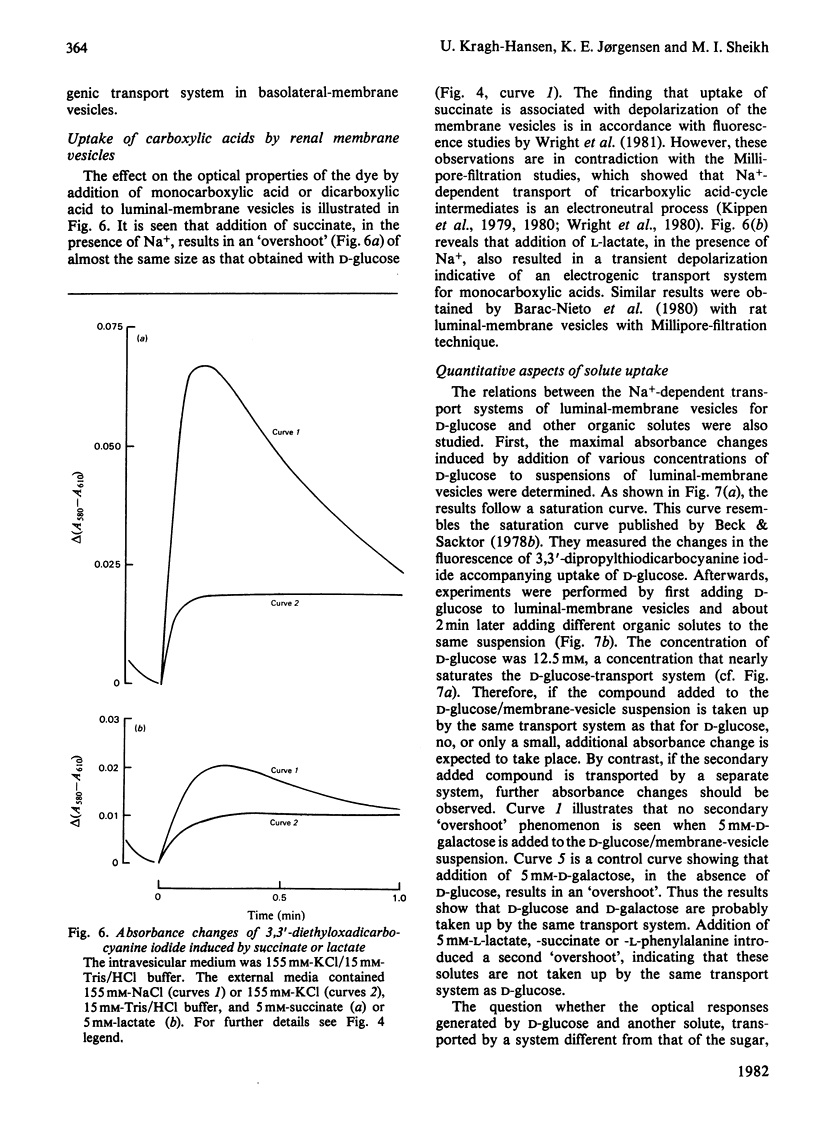
Abstract
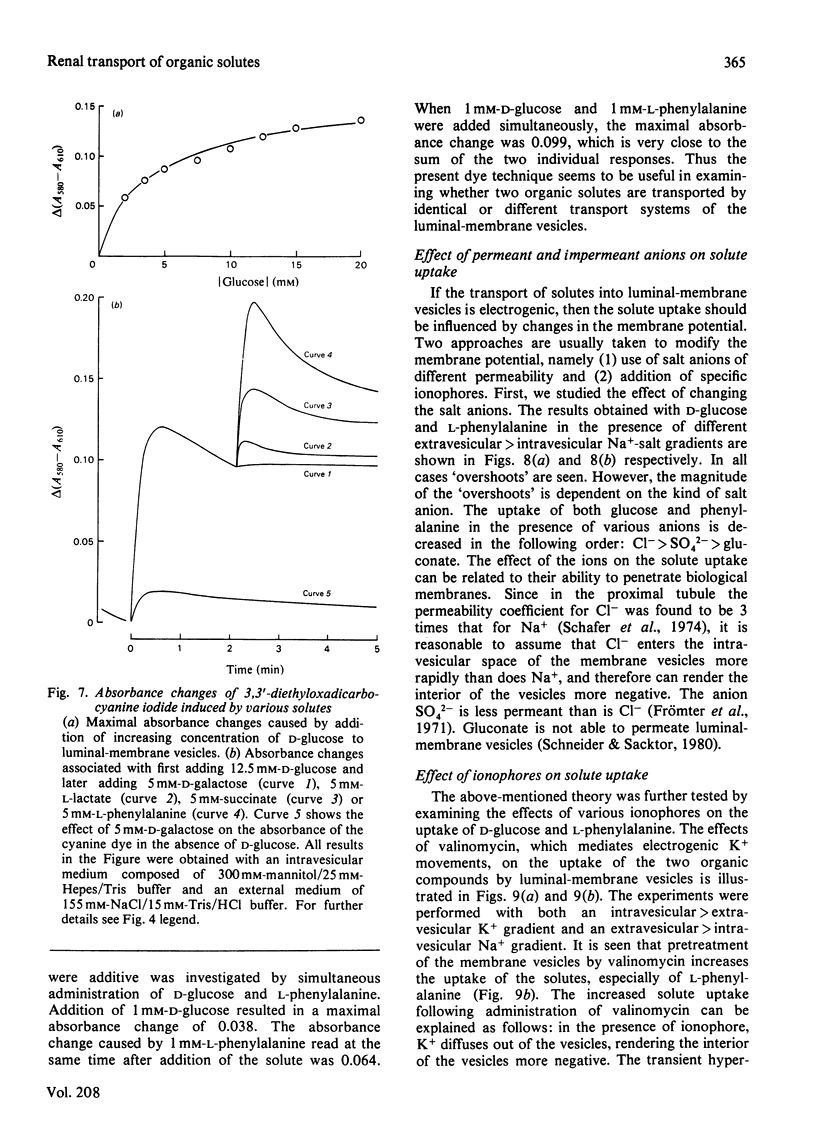
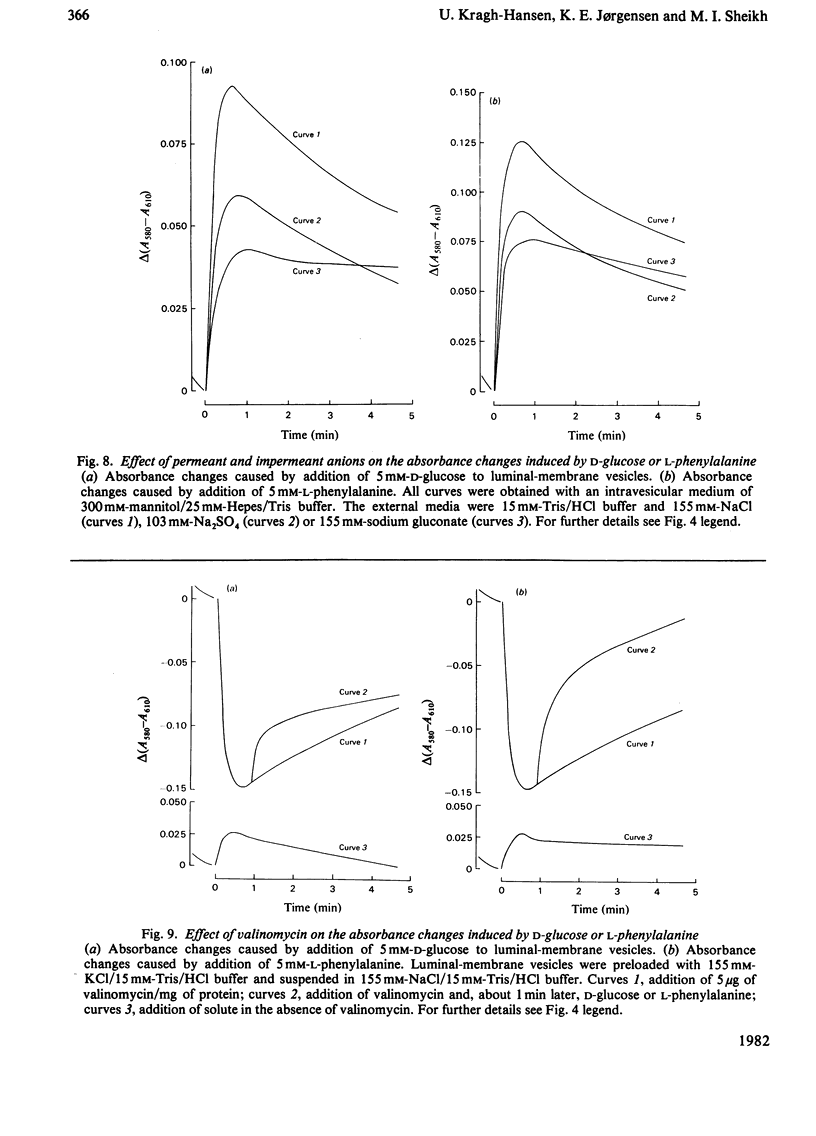
Renal transport of four different categories of organic solutes, namely sugars, neutral amino acids, monocarboxylic acids and dicarboxylic acids, was studied by using the potential-sensitive dye 3,3′-diethyloxadicarbocyanine iodide in purified luminal-membrane and basolateral-membrane vesicles isolated from rabbit kidney cortex. Valinomycin-induced K+ diffusion potentials resulted in concomitant changes in dye–membrane-vesicle absorption spectra. Linear relationships were obtained between these changes and depolarization and hyperpolarization of the vesicles. Addition of d-glucose, l-phenylalanine, succinate or l-lactate to luminal-membrane vesicles, in the presence of an extravesicular>intravesicular Na+ gradient, resulted in rapid transient depolarization. With basolateral-membrane vesicles no electrogenic transport of d-glucose or l-phenylalanine was observed. Spectrophotometric competition studies revealed that d-galactose is electrogenically taken up by the same transport system as that for d-glucose, whereas l-phenylalanine, succinate and l-lactate are transported by different systems in luminal-membrane vesicles. The absorbance changes associated with simultaneous addition of d-glucose and l-phenylalanine were additive. The uptake of these solutes was influenced by the presence of Na+-salt anions of different permeabilities in the order: Cl−>SO42−>gluconate. Addition of valinomycin to K+-loaded vesicles enhanced uptake of d-glucose and l-phenylalanine in the presence of an extravesicular>intravesicular Na+ gradient. Gramicidin or valinomycin plus nigericin diminished/abolished electrogenic solute uptake by Na+- or Na++K+-loaded vesicles respectively. These results strongly support the presence of Na+-dependent renal electrogenic transport of d-glucose, l-phenylalanine, succinate and l-lactate in luminal-membrane vesicles.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson P. S., Sacktor B. The Na+ gradient-dependent transport of D-glucose in renal brush border membranes. J Biol Chem. 1975 Aug 10;250(15):6032–6039. [PubMed] [Google Scholar]
- Barac-Nieto M., Murer H., Kinne R. Lactate-sodium cotransport in rat renal brush border membranes. Am J Physiol. 1980 Nov;239(5):F496–F506. doi: 10.1152/ajprenal.1980.239.5.F496. [DOI] [PubMed] [Google Scholar]
- Beck J. C., Sacktor B. Energetics of the Na+-dependent transport of D-glucose in renal brush border membrane vesicles. J Biol Chem. 1975 Nov 25;250(22):8674–8680. [PubMed] [Google Scholar]
- Beck J. C., Sacktor B. Membrane potential-sensitive fluorescence changes during Na+-dependent D-glucose transport in renal brush border membrane vesicles. J Biol Chem. 1978 Oct 25;253(20):7158–7162. [PubMed] [Google Scholar]
- Beck J. C., Sacktor B. The sodium electrochemical potential-mediated uphill transport of D-glucose in renal brush border membrane vesicles. J Biol Chem. 1978 Aug 10;253(15):5531–5535. [PubMed] [Google Scholar]
- Bennett N., Dupont Y. Evidence for a calcium-gated cation channel in sarcoplasmic reticulum vesicles. FEBS Lett. 1981 Jun 15;128(2):269–274. doi: 10.1016/0014-5793(81)80096-8. [DOI] [PubMed] [Google Scholar]
- Burckhardt G., Kinne R., Stange G., Murer H. The effects of potassium and membrane potential on sodium-dependent glutamic acid uptake. Biochim Biophys Acta. 1980 Jun 20;599(1):191–201. doi: 10.1016/0005-2736(80)90067-x. [DOI] [PubMed] [Google Scholar]
- Douglas M. G., Cockrell R. S. Mitochondrial cation-hydrogen ion exchange. Sodium selective transport by mitochondria and submitochondrial particles. J Biol Chem. 1974 Sep 10;249(17):5464–5471. [PubMed] [Google Scholar]
- Evers C., Haase W., Murer H., Kinne R. Properties of brush border vesicles isolated from rat kidney cortex by calcium precipitation. Membr Biochem. 1978;1(3-4):203–219. doi: 10.3109/09687687809063848. [DOI] [PubMed] [Google Scholar]
- Evers J., Murer H., Kinne R. Phenylalanine uptake in isolated renal brush border vesicles. Biochim Biophys Acta. 1976 Apr 5;426(4):598–615. doi: 10.1016/0005-2736(76)90124-3. [DOI] [PubMed] [Google Scholar]
- Guillet E. G., Kimmich G. A. DiO-C3-(5) and DiS-C3-(5): Interactions with RBC, ghosts and phospholipid vesicles. J Membr Biol. 1981 Mar 15;59(1):1–11. doi: 10.1007/BF01870815. [DOI] [PubMed] [Google Scholar]
- Gupta R. K., Salzberg B. M., Grinvald A., Cohen L. B., Kamino K., Lesher S., Boyle M. B., Waggoner A. S., Wang C. H. Improvements in optical methods for measuring rapid changes in membrane potential. J Membr Biol. 1981 Feb 15;58(2):123–137. doi: 10.1007/BF01870975. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Transport in isolated bacterial membrane vesicles. Methods Enzymol. 1974;31:698–709. doi: 10.1016/0076-6879(74)31075-0. [DOI] [PubMed] [Google Scholar]
- Kinnally K. W., Tedeschi H., Maloff B. L. Use of dyes to estimate the electrical potential of the mitochondrial membrane. Biochemistry. 1978 Aug 8;17(16):3419–3428. doi: 10.1021/bi00609a036. [DOI] [PubMed] [Google Scholar]
- Kinsella J. L., Holohan P. D., Pessah N. I., Ross C. R. Isolation of luminal and antiluminal membranes from dog kidney cortex. Biochim Biophys Acta. 1979 Apr 19;552(3):468–477. doi: 10.1016/0005-2736(79)90191-3. [DOI] [PubMed] [Google Scholar]
- Kippen I., Hirayama B., Klinenberg J. R., Wright E. M. Transport of tricarboxylic acid cycle intermediates by membrane vesicles from renal brush border. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3397–3400. doi: 10.1073/pnas.76.7.3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laris P. C., Bootman M., Pershadsingh H. A., Johnstone R. M. The influence of cellular amino acids and the Na+ : K+ pump on the membrane potential of the Ehrlich ascites tumor cell. Biochim Biophys Acta. 1978 Sep 22;512(2):397–414. doi: 10.1016/0005-2736(78)90263-8. [DOI] [PubMed] [Google Scholar]
- Meissner G., Young R. C. Proton permeability of sarcoplasmic reticulum vesicles. J Biol Chem. 1980 Jul 25;255(14):6814–6819. [PubMed] [Google Scholar]
- Murer H., Kinne R. The use of isolated membrane vesicles to study epithelial transport processes. J Membr Biol. 1980 Jul 15;55(2):81–95. doi: 10.1007/BF01871151. [DOI] [PubMed] [Google Scholar]
- Pershadsingh H. A., Johnstone R. M., Laris P. C. Influence of (DL)-propranolol and Ca2+ on membrane potential and amino acid transport in Ehrlich ascites tumor cells. Biochim Biophys Acta. 1978 May 18;509(2):360–373. doi: 10.1016/0005-2736(78)90054-8. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Schafer J. A., Troutman S. L., Andreoli T. E. Volume reabsorption, transepithelial potential differences, and ionic permeability properties in mammalian superficial proximal straight tubules. J Gen Physiol. 1974 Nov;64(5):582–607. doi: 10.1085/jgp.64.5.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E. G., Sacktor B. Sodium gradient-dependent L-glutamate transport in renal brush border membrane vesicles. Effect of an intravesicular > extravesicular potassium gradient. J Biol Chem. 1980 Aug 25;255(16):7645–7649. [PubMed] [Google Scholar]
- Sheikh M. I., Kragh-Hansen U., Jørgensen K. E., Røigaard-Petersen H. An efficient method for the isolation and separation of basolateral-membrane and luminal-membrane vesicles from rabbit kidney cortex. Biochem J. 1982 Nov 15;208(2):377–382. doi: 10.1042/bj2080377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. C., Frank S. J., Bashford C. L., Chance B., Rudkin B. Kinetics of the association of potential-sensitive dyes with model and energy-transducing membranes: implications for fast probe response times. J Membr Biol. 1980 May 23;54(2):127–139. doi: 10.1007/BF01940566. [DOI] [PubMed] [Google Scholar]
- Wright S. H., Kippen I., Klinenberg J. R., Wright E. M. Specificity of the transport system for tricarboxylic acid cycle intermediates in renal brush borders. J Membr Biol. 1980 Nov 15;57(1):73–82. doi: 10.1007/BF01868987. [DOI] [PubMed] [Google Scholar]
- Wright S. H., Krasne S., Kippen I., Wright E. M. Na+-dependent transport of tricarboxylic acid cycle intermediates by renal brush border membranes. Effects on fluorescence of a potential-sensitive cyanine dye. Biochim Biophys Acta. 1981 Feb 6;640(3):767–778. doi: 10.1016/0005-2736(81)90107-3. [DOI] [PubMed] [Google Scholar]


