Abstract
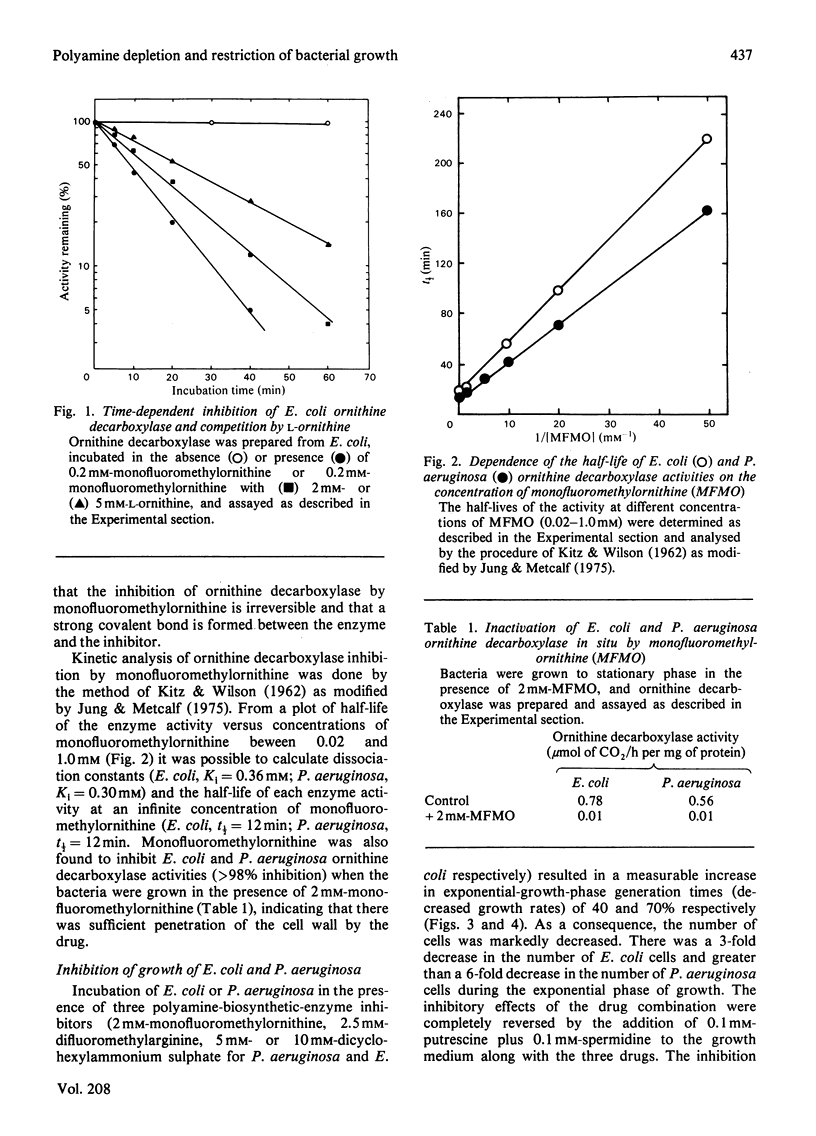
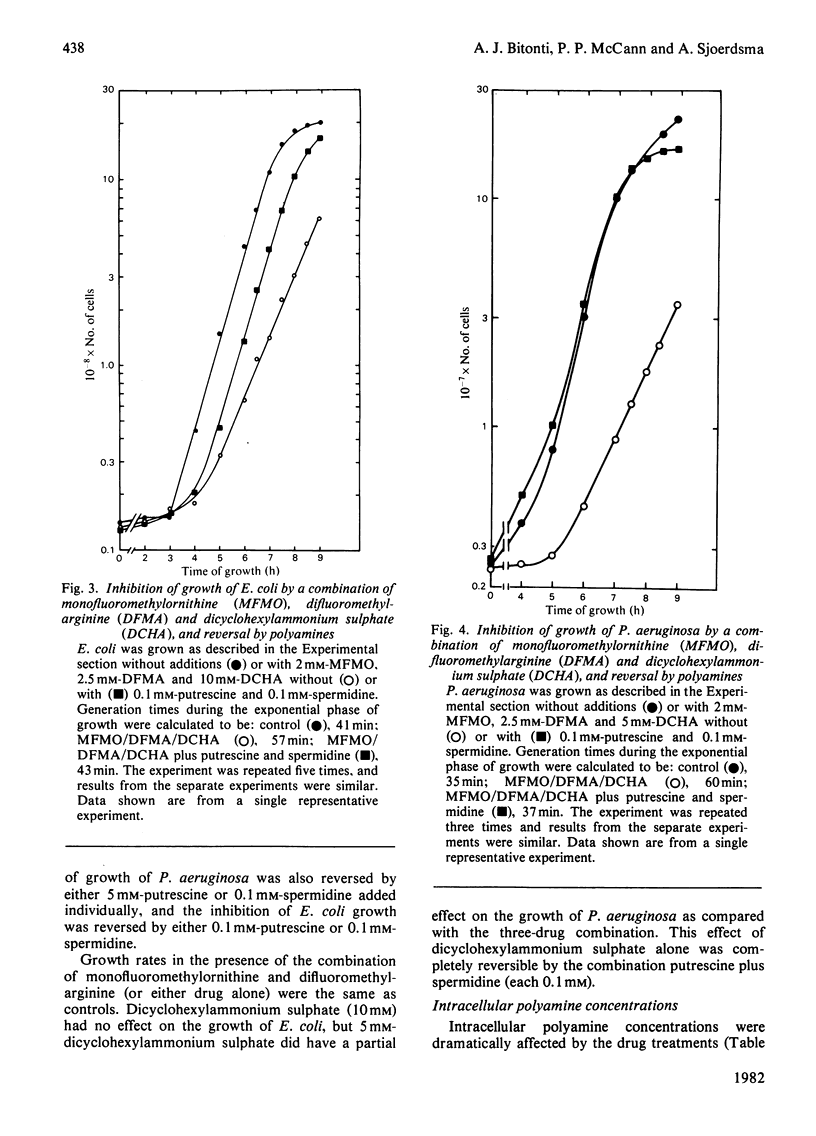
Bacterial growth was measurably slowed by a combination of drugs which inhibit polyamine-biosynthetic enzymes. Addition of DL-alpha-monofluoromethylornithine, which was shown to inactivate irreversibly ornithine decarboxylase extracted from Escherichia coli (Ki = 0.36 mM) and Pseudomonas aeruginosa (Ki = 0.30 mM), DL-alpha-difluoromethylarginine and dicyclohexylammonium sulphate to cultures of E. coli or P. aeruginosa resulted in a 40 and 70% increase in generation times (decreased growth rates) respectively, which was completely reversed by the addition of 0.1 mM-putrescine plus 0.1 mM-spermidine to the medium. Decreased intracellular polyamine concentrations correlated with increased generation times; putrescine concentration was decreased by 70% in E. coli and 80% in P. aeruginosa, while spermidine concentration was decreased by 50% in E. coli and 95% in P. aeruginosa. Subsequent investigation of the inactivation of the ornithine decarboxylase by monofluoromethylornithine indicated that it was active-site directed, as the normal substrate ornithine slowed the rate of inhibition. Specific interference with polyamine biosynthesis may be a viable approach to control of some bacterial infections.
Full text
PDF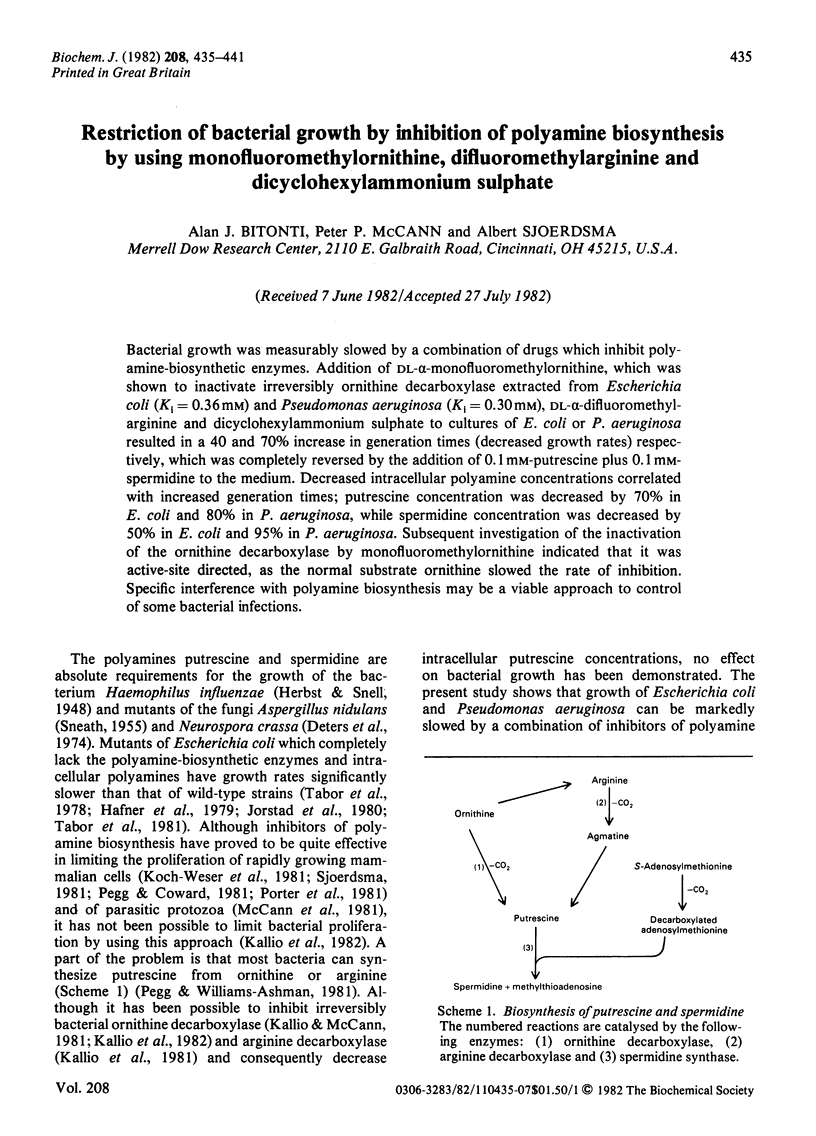




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERTON J. I., LOCKE D. J. Extraction of pigment from cooked cured-meat products. Nature. 1955 May 7;175(4462):818–819. doi: 10.1038/175818b0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner E. W., Tabor C. W., Tabor H. Mutants of Escherichia coli that do not contain 1,4-diaminobutane (putrescine) or spermidine. J Biol Chem. 1979 Dec 25;254(24):12419–12426. [PubMed] [Google Scholar]
- Hibasami H., Tanaka M., Nagai J., Ikeda T. Dicyclohexylamine, a potent inhibitor of spermidine synthase in mammalian cells. FEBS Lett. 1980 Jul 11;116(1):99–101. doi: 10.1016/0014-5793(80)80537-0. [DOI] [PubMed] [Google Scholar]
- Jorstad C. M., Harada J. J., Morris D. R. Structural specificity of the spermidine requirement of an Escherichia coli auxotroph. J Bacteriol. 1980 Feb;141(2):456–463. doi: 10.1128/jb.141.2.456-463.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M. J., Metcalf B. W. Catalytic inhibition of gamma-aminobutyric acid - alpha-ketoglutarate transaminase of bacterial origin by 4-aminohex-5-ynoic acid, a substrate analog. Biochem Biophys Res Commun. 1975 Nov 3;67(1):301–306. doi: 10.1016/0006-291x(75)90316-2. [DOI] [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. On the purification of L-ornithine decarboxylase from rat prostate and effects of thiol compounds on the enzyme. J Biol Chem. 1971 Mar 25;246(6):1725–1732. [PubMed] [Google Scholar]
- KITZ R., WILSON I. B. Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase. J Biol Chem. 1962 Oct;237:3245–3249. [PubMed] [Google Scholar]
- Kallio A., McCann P. P., Bey P. DL-a-Monofluoromethylputrescine is a potent irreversible inhibitor of Escherichia coli ornithine decarboxylase. Biochem J. 1982 Jun 15;204(3):771–775. doi: 10.1042/bj2040771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio A., McCann P. P., Bey P. DL-alpha-(Difluoromethyl)arginine: a potent enzyme-activated irreversible inhibitor of bacterial decarboxylases. Biochemistry. 1981 May 26;20(11):3163–3168. doi: 10.1021/bi00514a027. [DOI] [PubMed] [Google Scholar]
- Kallio A., McCann P. P. Difluoromethylornithine irreversibly inactivates ornithine decarboxylase of Pseudomonas aeruginosa, but does not inhibit the enzymes of Escherichia coli. Biochem J. 1981 Oct 15;200(1):69–75. doi: 10.1042/bj2000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollonitsch J., Perkins L. M., Patchett A. A., Doldouras G. A., Marburg S., Duggan D. E., Maycock A. L., Aster S. D. Selective inhibitors of biosynthesis of aminergic neurotransmitters. Nature. 1978 Aug 31;274(5674):906–908. doi: 10.1038/274906a0. [DOI] [PubMed] [Google Scholar]
- Sjoerdsma A. Suicide enzyme inhibitors as potential drugs. Clin Pharmacol Ther. 1981 Jul;30(1):3–22. doi: 10.1038/clpt.1981.121. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H., Hafner E. W. Escherichia coli mutants completely deficient in adenosylmethionine decarboxylase and in spermidine biosynthesis. J Biol Chem. 1978 May 25;253(10):3671–3676. [PubMed] [Google Scholar]
- Tabor H., Tabor C. W., Cohn M. S., Hafner E. W. Streptomycin resistance (rpsL) produces an absolute requirement for polyamines for growth of an Escherichia coli strain unable to synthesize putrescine and spermidine [delta(speA-speB) delta specC]. J Bacteriol. 1981 Aug;147(2):702–704. doi: 10.1128/jb.147.2.702-704.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


