Abstract
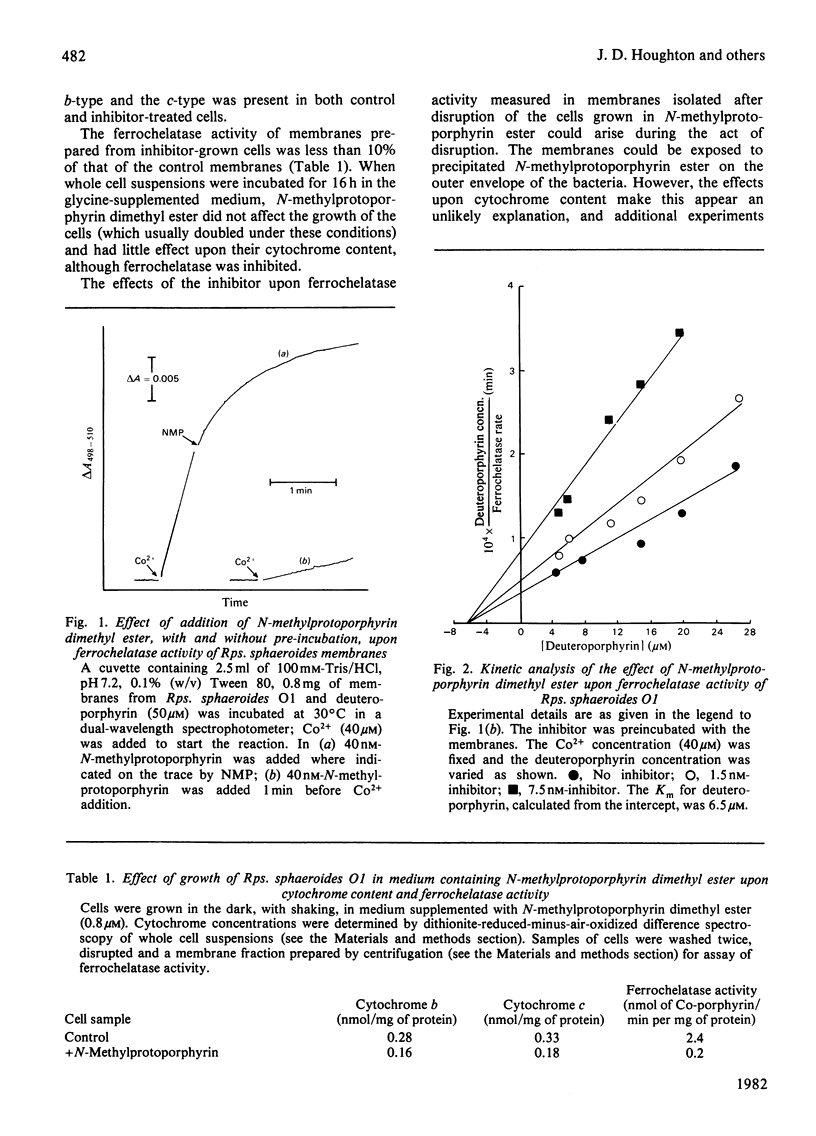
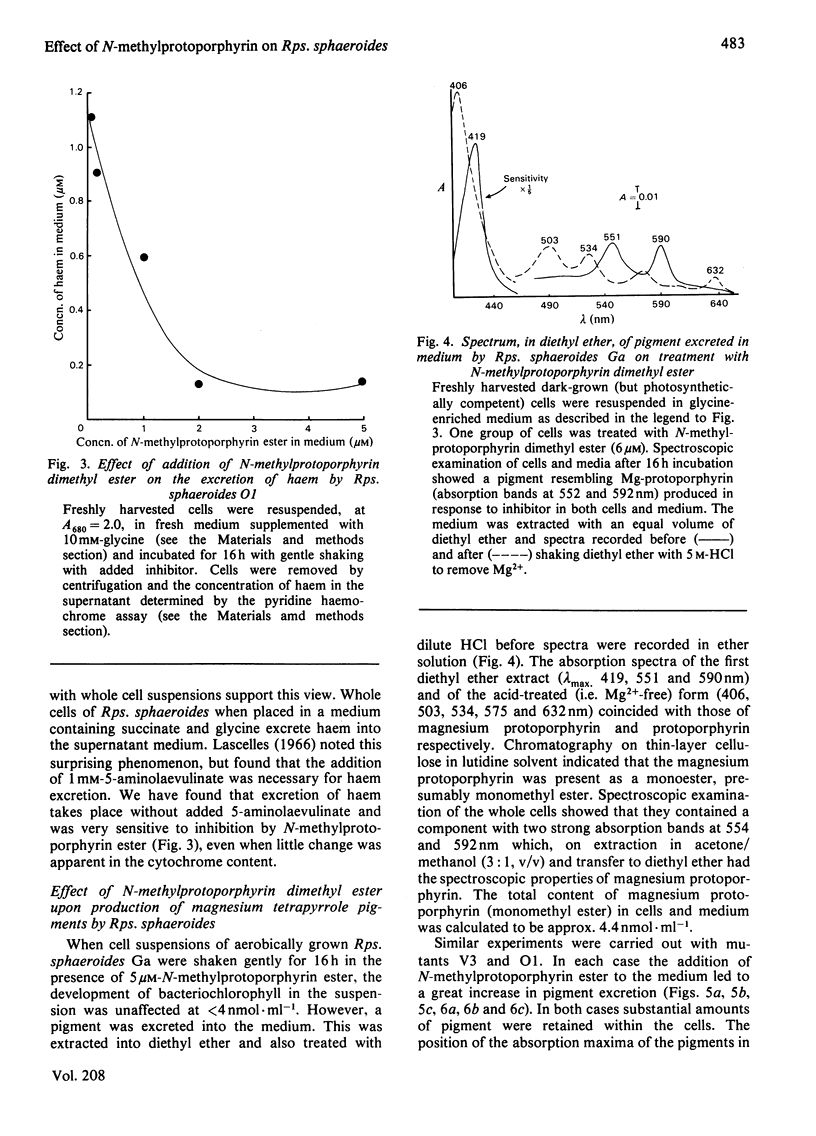
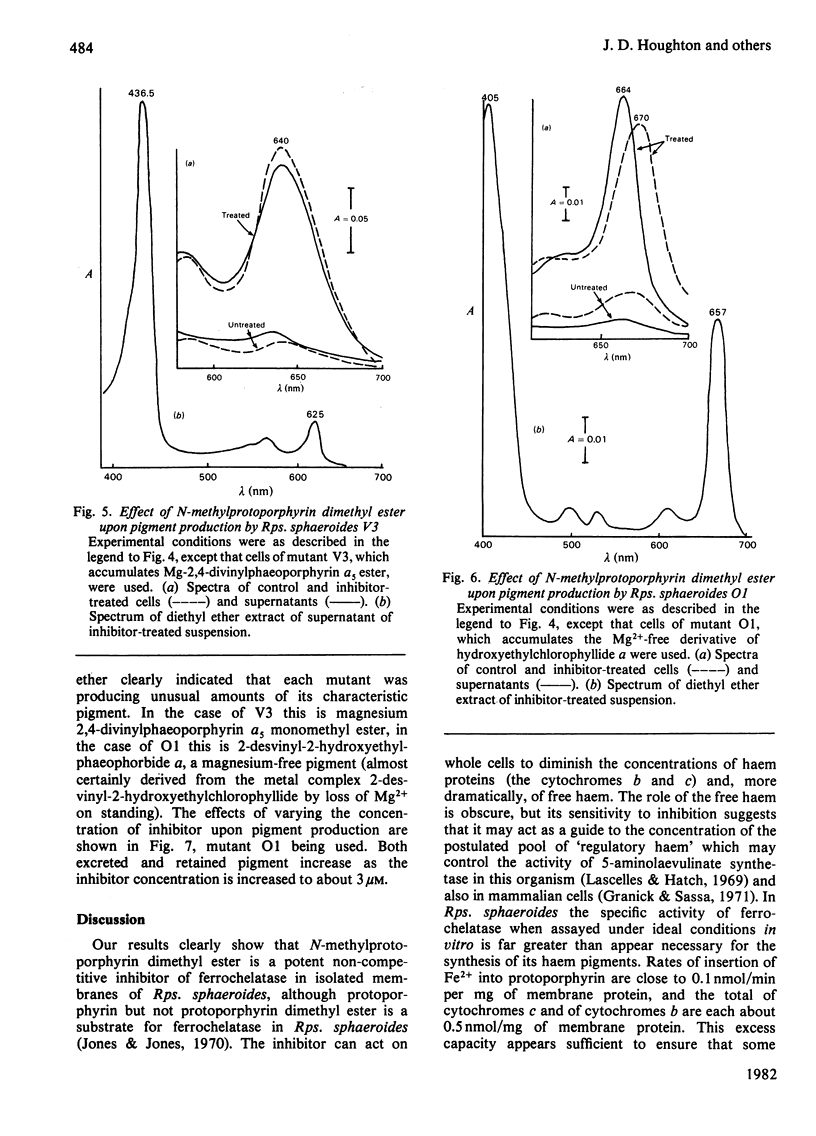
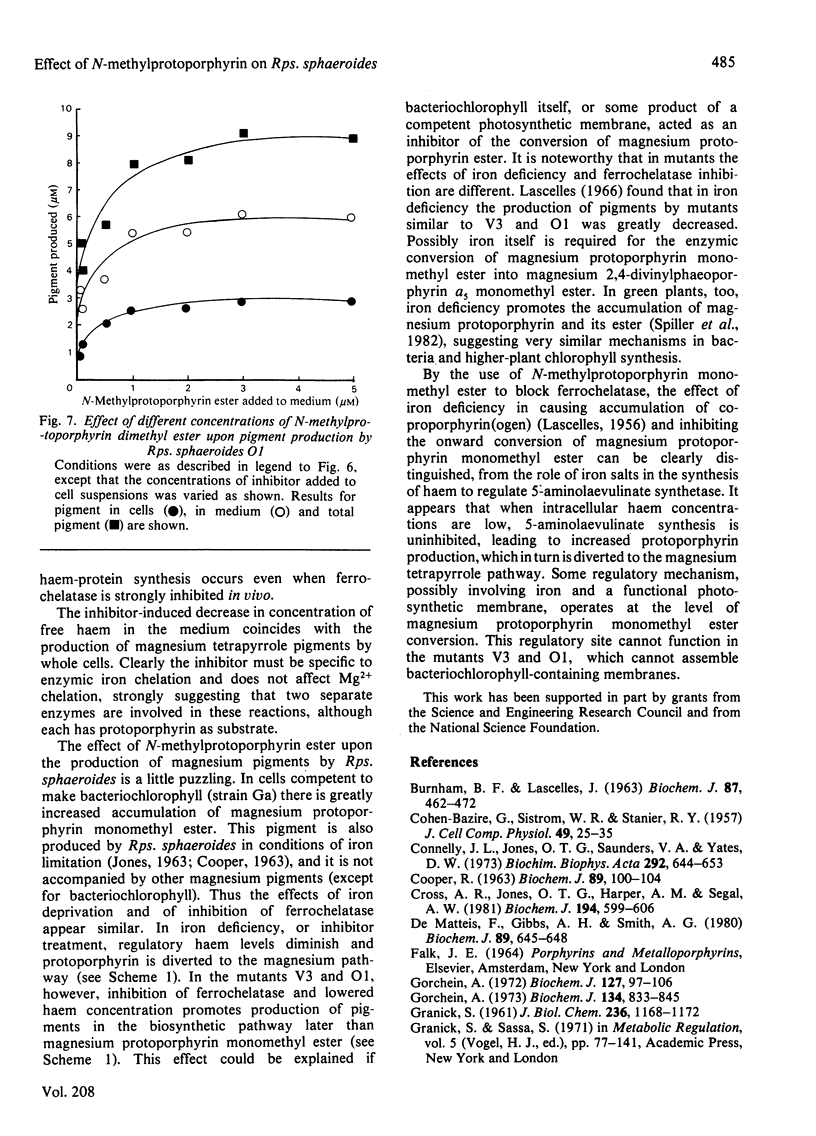
N-Methylprotoporphyrin dimethyl ester inhibits ferrochelatase in isolated membranes of Rhodopseudomonas sphaeroides at low concentrations (around 10 nm). Full inhibition developed after a short lag phase. The inhibition was non-competitive with porphyrin substrate. Addition of inhibitor to growing cultures of Rps. sphaeroides caused a decrease (near 40%) in cytochrome content and a severe inhibition of ferrochelatase; the excretion of haem into the medium by cell suspensions was also severely inhibited. The addition of N-methylprotoporphyrin dimethyl ester to suspensions of photosynthetically competent Rps. sphaeroides Ga caused excretion of Mg-protoporphyrin monomethyl ester. When added to mutants V3 and O1, magnesium divinylphaeoporphyrin a5 monomethyl ester and 2-devinyl-2-hydroxyethylphaeophorbide a were excreted, with maximum effect at around 3 microM-inhibitor in the medium. The results are interpreted to suggest that the inhibitor decreases concentration of intracellular haem, which normally controls the activity of 5-aminolaevulinate synthetase. Unregulated activity of this enzyme leads to overproduction of protoporphyrin, which is diverted to the bacteriochlorophyll pathway. Further control operates at magnesium protoporphyrin ester conversion in normal cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNHAM B. F., PIERCE W. S., WILLIAMS K. R., BOYER M. H., KIRBY C. K. delta-aminolaevulate dehydratase from Rhodopseudomonas spheroides. Biochem J. 1963 Jun;87:462–472. doi: 10.1042/bj0870462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- COOPER R. THE BIOSYNTHESIS OF COPROPORPHYRINOGEN, MAGNESIUM PROTOPORPHYRIN MONOMETHYL ESTER AND BACTERIOCHLOROPHYLL BY RHODOPSEUDOMONAS CAPSULATA. Biochem J. 1963 Oct;89:100–108. doi: 10.1042/bj0890100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly J. L., Jones O. T., Saunders V. A., Yates D. W. Kinetic and thermodynamic properties of membrane-bound cytochromes of aerobically and photosynthetically grown Rhodopseudomonas spheroides. Biochim Biophys Acta. 1973 Apr 5;292(3):644–653. doi: 10.1016/0005-2728(73)90012-1. [DOI] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T., Harper A. M., Segal A. W. Oxidation-reduction properties of the cytochrome b found in the plasma-membrane fraction of human neutrophils. A possible oxidase in the respiratory burst. Biochem J. 1981 Feb 15;194(2):599–606. doi: 10.1042/bj1940599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F., Gibbs A. H., Smith A. G. Inhibition of protohaem ferro-lyase by N-substituted porphyrins. Structural requirements for the inhibitory effect. Biochem J. 1980 Sep 1;189(3):645–648. doi: 10.1042/bj1890645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANICK S. Magnesium protoporphyrin monoester and protoporphyrin monomethyl ester in chlorophyll biosynthesis. J Biol Chem. 1961 Apr;236:1168–1172. [PubMed] [Google Scholar]
- Gorchein A. Control of magnesium-protoporphyrin chelatase activity in Rhodopseudomonas spheroides. Role of light, oxygen, and electron and energy transfer. Biochem J. 1973 Aug;134(4):833–845. doi: 10.1042/bj1340833d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorchein A. Magnesium protoporphyrin chelatase activity in Rhodopseudomonas spheroides. Studies with whole cells. Biochem J. 1972 Mar;127(1):97–106. doi: 10.1042/bj1270097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES O. T. The production of magnesium protoporphyrin monomethyl ester by Rhodopseudomonas spheroides. Biochem J. 1963 Mar;86:429–432. doi: 10.1042/bj0860429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. S., Jones O. T. Ferrochelatase of Rhodopseudomonas spheroides. Biochem J. 1970 Sep;119(3):453–462. doi: 10.1042/bj1190453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. S., Jones O. T. The structural organization of haem synthesis in rat liver mitochondria. Biochem J. 1969 Jul;113(3):507–514. doi: 10.1042/bj1130507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones O. T. Studies on the structure of a pigment related to chlorophyll a produced by Rhodopseudomonas spheroides. Biochem J. 1964 Jun;91(3):572–576. doi: 10.1042/bj0910572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASCELLES J. The synthesis of porphyrins and bacteriochlorophyll by cell suspensions of Rhodopseudomonas spheroides. Biochem J. 1956 Jan;62(1):78–93. doi: 10.1042/bj0620078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascelles J., Hatch T. P. Bacteriochlorophyll and heme synthesis in Rhodopseudomonas spheroides: possible role of heme in regulation of the branched biosynthetic pathway. J Bacteriol. 1969 May;98(2):712–720. doi: 10.1128/jb.98.2.712-720.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascelles J. The accumulation of bacteriochlorophyll precursors by mutant and wild-type strains of Rhodopseudomonas spheroides. Biochem J. 1966 Jul;100(1):175–183. doi: 10.1042/bj1000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SISTROM W. R. A requirement for sodium in the growth of Rhodopseudomonas spheroides. J Gen Microbiol. 1960 Jun;22:778–785. doi: 10.1099/00221287-22-3-778. [DOI] [PubMed] [Google Scholar]
- Spiller S. C., Castelfranco A. M., Castelfranco P. A. Effects of Iron and Oxygen on Chlorophyll Biosynthesis : I. IN VIVO OBSERVATIONS ON IRON AND OXYGEN-DEFICIENT PLANTS. Plant Physiol. 1982 Jan;69(1):107–111. doi: 10.1104/pp.69.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tephly T. R., Coffman B. L., Ingall G., Ziet-Har M. S., Goff H. M., Tabba H. D., Smith K. M. Identification of N-methylprotoporphyrin IX in livers of untreated mice and mice treated with 3, 5-diethoxycarbonyl- 1, 4-dihydrocollidine: source of the methyl group. Arch Biochem Biophys. 1981 Nov;212(1):120–126. doi: 10.1016/0003-9861(81)90350-7. [DOI] [PubMed] [Google Scholar]


