Abstract
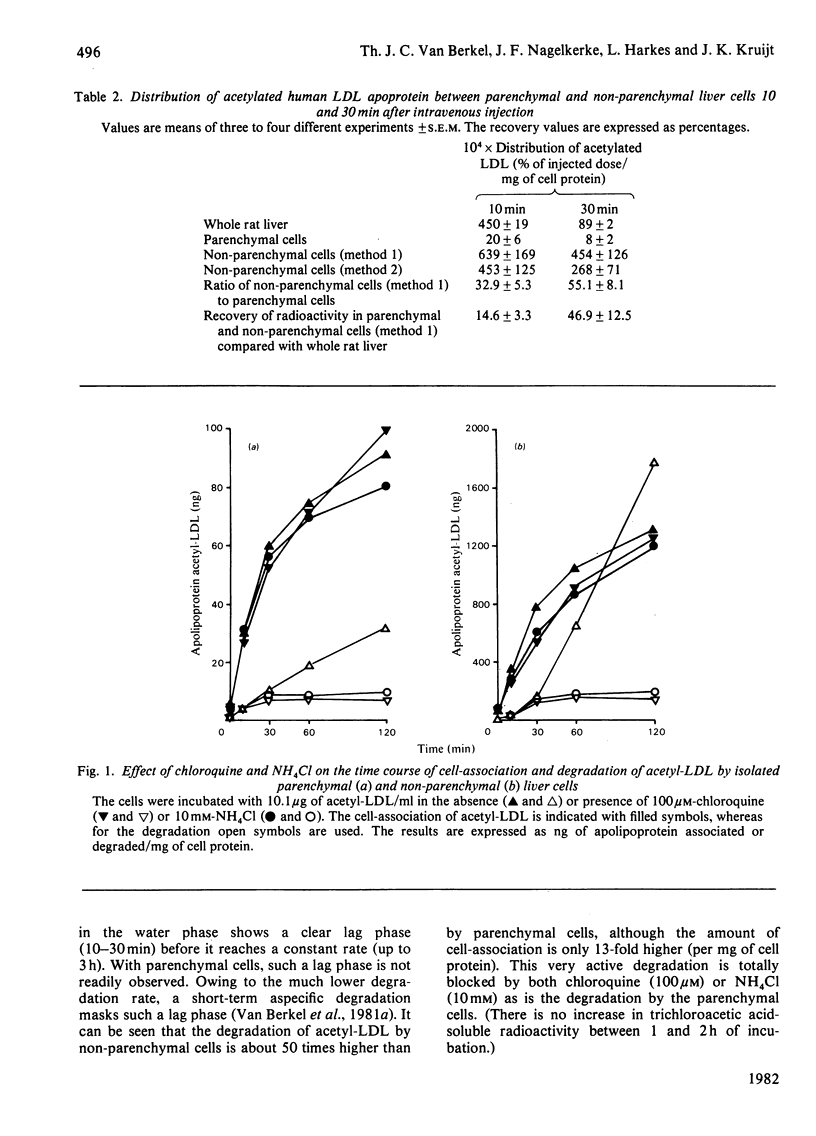
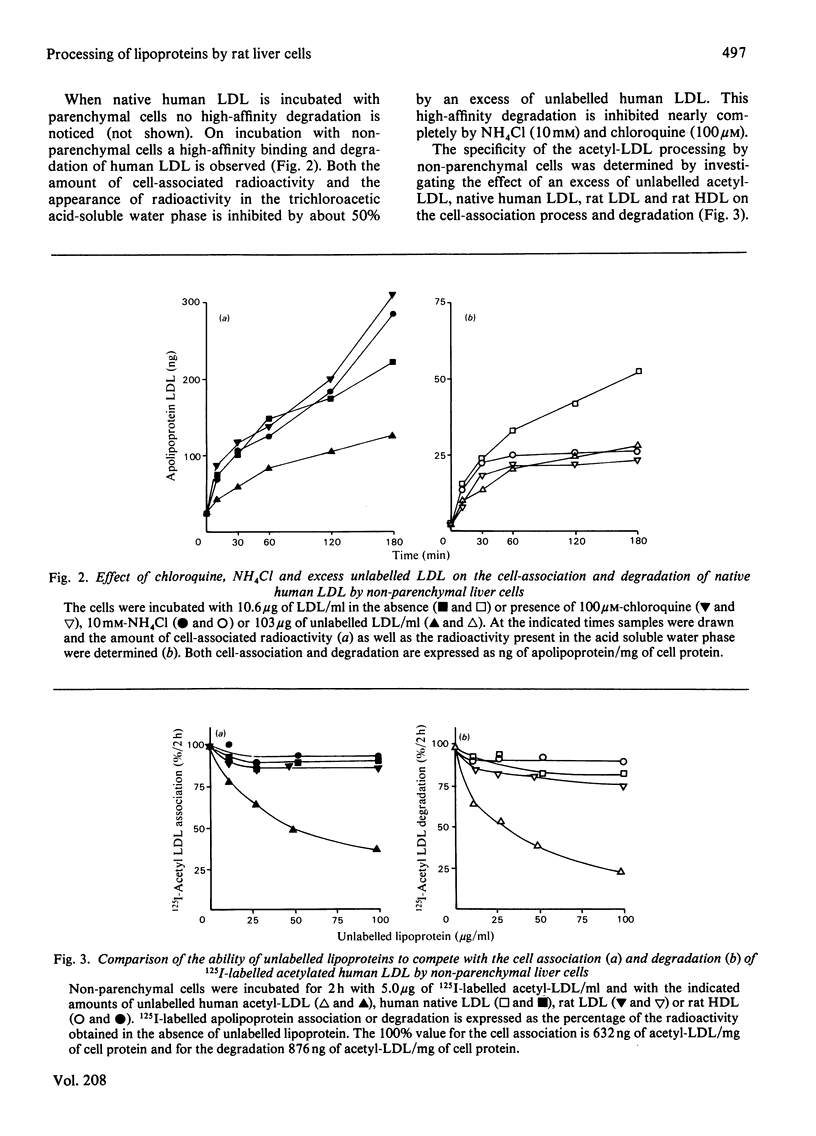
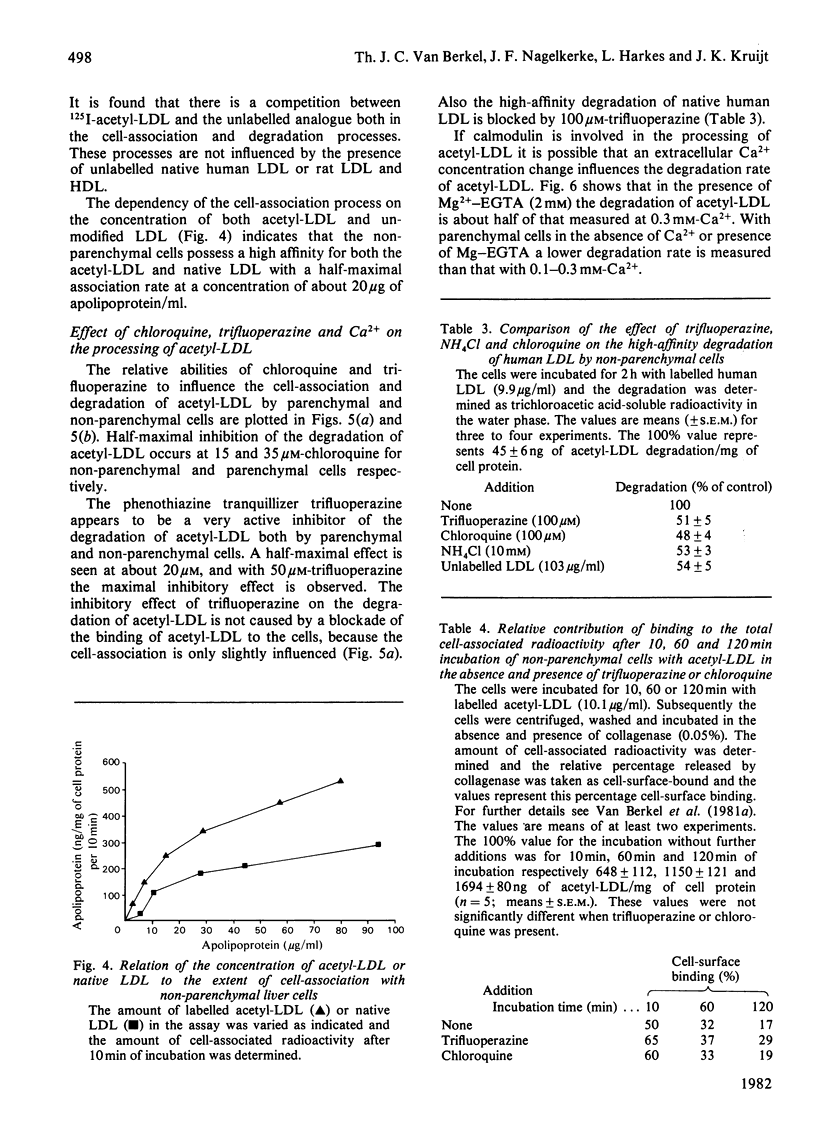
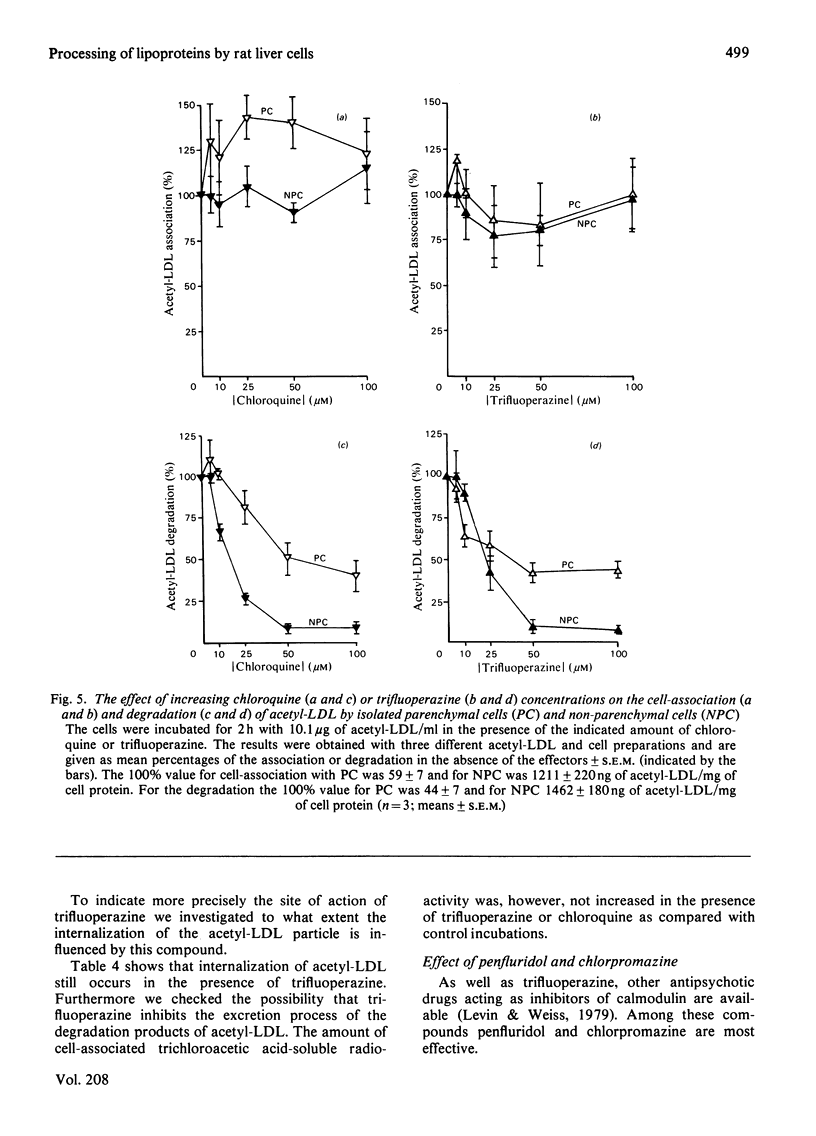
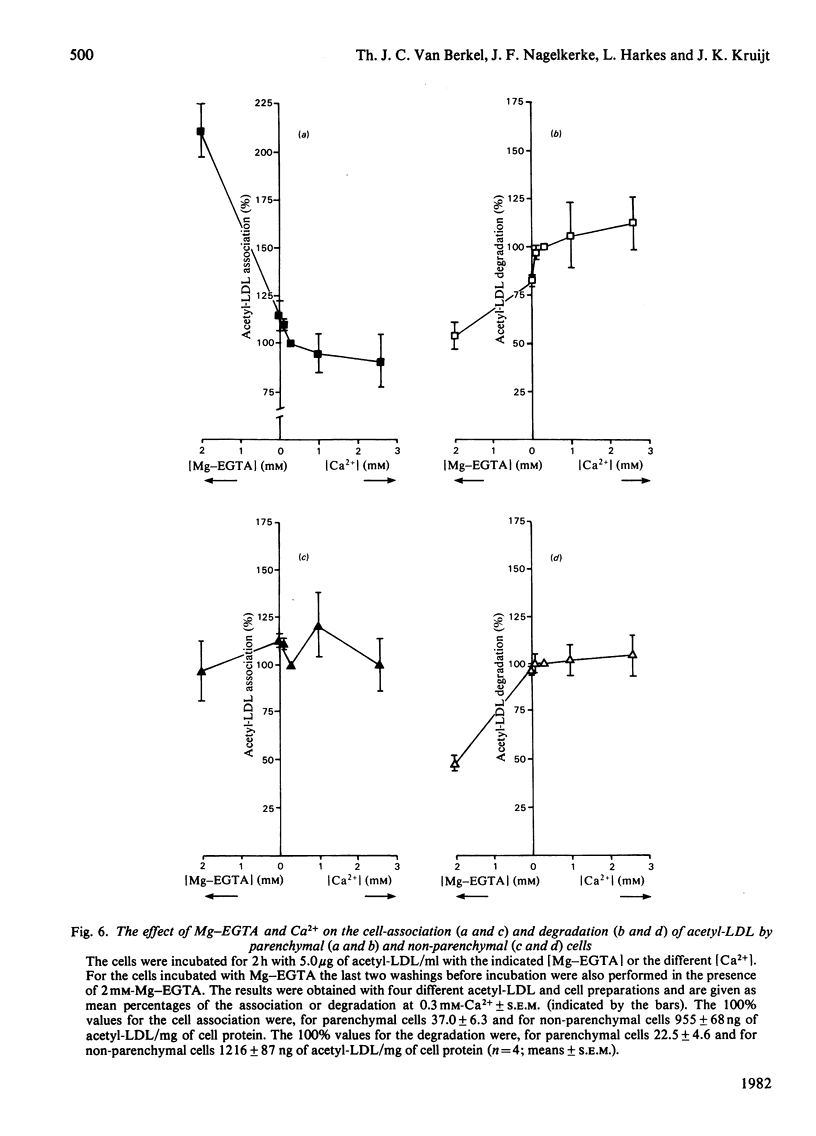
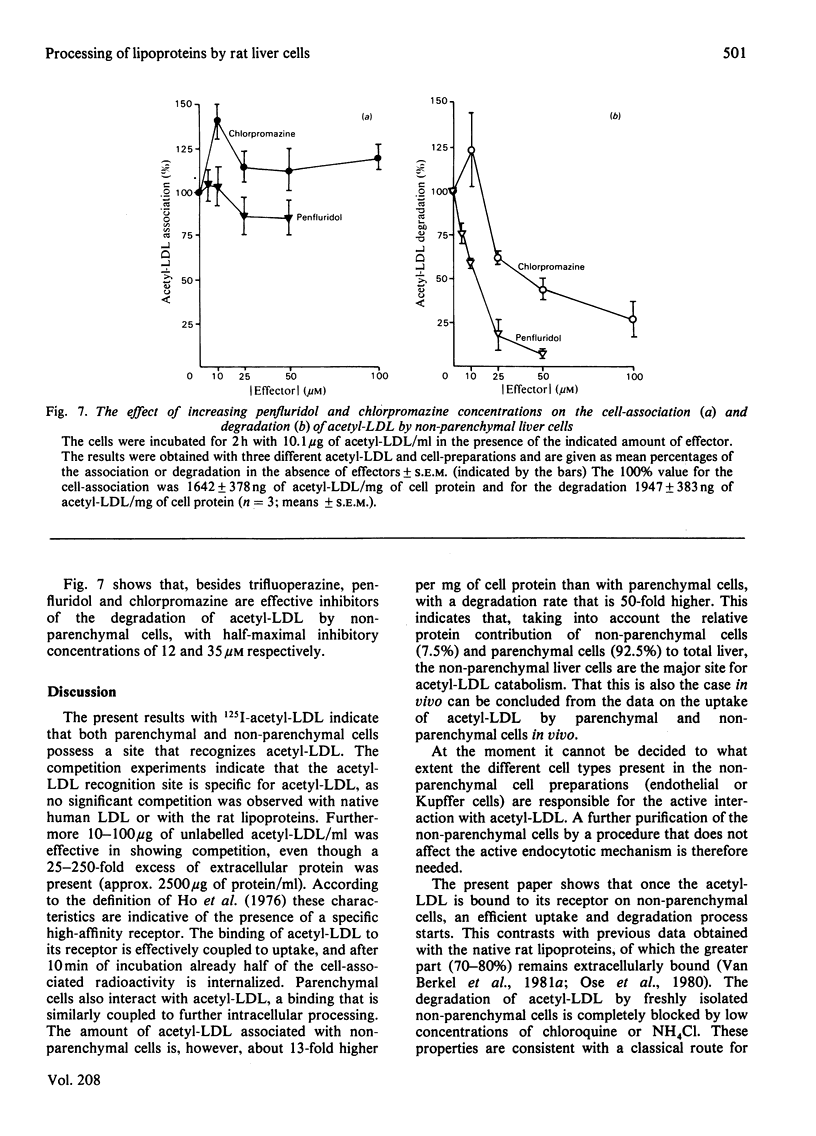
1. Modified lipoproteins have been implicated to play a significant role in the pathogenesis of atherosclerosis. In view of this we studied the fate and mechanism of uptake in vivo of acetylated human low-density lipoprotein (acetyl-LDL). Injected intravenously into rats, acetyl-LDL is rapidly cleared from the blood. At 10min after intravenous injection, 83% of the injected dose is recovered in liver. Separation of the liver into a parenchymal and non-parenchymal cell fraction indicates that the non-parenchymal cells contain a 30–50-fold higher amount of radioactivity per mg of cell protein than the parenchymal cells. 2. When incubated in vitro, freshly isolated non-parenchymal cells show a cell-association of acetyl-LDL that is 13-fold higher per mg of cell protein than with parenchymal cells, and the degradation of acetyl-LDL is 50-fold higher. The degradation of acetyl-LDL by both cell types is blocked by chloroquine (10–50μm) and NH4Cl (10mm), indicating that it occurs in the lysosomes. Competition experiments indicate the presence of a specific acetyl-LDL receptor and degradation pathway, which is different from that for native LDL. 3. Degradation of acetyl-LDL by non-parenchymal cells is completely blocked by trifluoperazine, penfluridol and chlorpromazine with a relative effectivity that corresponds to their effectivity as calmodulin inhibitors. The high-affinity degradation of human LDL is also blocked by trifluoperazine (100μm). The inhibition of the processing of acetyl-LDL occurs at a site after the binding-internalization process and before intralysosomal degradation. It is suggested that calmodulin, or a target with a similar sensitivity to calmodulin inhibitors, is involved in the transport of the endocytosed acetyl-LDL to or into the lysosomes. 4. It is concluded that the liver, and in particular non-parenchymal liver cells, are in vivo the major site for acetyl-LDL uptake. This efficient uptake and degradation mechanism for acetyl-LDL in the liver might form in vivo the major protection system against the potential pathogenic action of modified lipoproteins.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basu S. K., Goldstein J. L., Anderson G. W., Brown M. S. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3178–3182. doi: 10.1073/pnas.73.9.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierman E. L., Stein O., Stein Y. Lipoprotein uptake and metabolism by rat aortic smooth muscle cells in tissue culture. Circ Res. 1974 Jul;35(1):136–150. doi: 10.1161/01.res.35.1.136. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Ho Y. K., Goldstein J. L. The cholesteryl ester cycle in macrophage foam cells. Continual hydrolysis and re-esterification of cytoplasmic cholesteryl esters. J Biol Chem. 1980 Oct 10;255(19):9344–9352. [PubMed] [Google Scholar]
- Chomette G., de Gennes J. L., Delcourt A., Hammou J. C., Perie G. La xanthomatose cutanéo-tendineuse hypercholestérolémique familiale. Etude anatomo-clinique. A propos de deux cas. Ann Anat Pathol (Paris) 1971 Jul-Sep;16(3):233–250. [PubMed] [Google Scholar]
- Crisp D. M., Pogson C. I. Glycolytic and gluconeogenic enzyme activities in parenchymal and non-parenchymal cells from mouse liver. Biochem J. 1972 Feb;126(4):1009–1023. doi: 10.1042/bj1261009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelman A. M., Shechter I., Seager J., Hokom M., Child J. S., Edwards P. A. Malondialdehyde alteration of low density lipoproteins leads to cholesteryl ester accumulation in human monocyte-macrophages. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2214–2218. doi: 10.1073/pnas.77.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Familial hypercholesterolemia: pathogenesis of a receptor disease. Johns Hopkins Med J. 1978 Jul;143(1):8–16. [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot P. H., Van Berkel T. J., Van Tol A. Relative contributions of parenchymal and non-parenchymal (sinusoidal) liver cells in the uptake of chylomicron remnants. Metabolism. 1981 Aug;30(8):792–797. doi: 10.1016/0026-0495(81)90025-1. [DOI] [PubMed] [Google Scholar]
- Henriksen T., Mahoney E. M., Steinberg D. Enhanced macrophage degradation of low density lipoprotein previously incubated with cultured endothelial cells: recognition by receptors for acetylated low density lipoproteins. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6499–6503. doi: 10.1073/pnas.78.10.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y. K., Brown S., Bilheimer D. W., Goldstein J. L. Regulation of low density lipoprotein receptor activity in freshly isolated human lymphocytes. J Clin Invest. 1976 Dec;58(6):1465–1474. doi: 10.1172/JCI108603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knook D. L., Sleyster E. C. Separation of Kupffer and endothelial cells of the rat liver by centrifugal elutriation. Exp Cell Res. 1976 May;99(2):444–449. doi: 10.1016/0014-4827(76)90605-4. [DOI] [PubMed] [Google Scholar]
- Kooistra T., Duursma A. M., Bouma J. M., Gruber M. Endocytosis and breakdown of ribonuclease oligomers by sinusoidal rat liver cells in vivo. I. Effect of size. Biochim Biophys Acta. 1979 Oct 4;587(2):282–298. doi: 10.1016/0304-4165(79)90361-1. [DOI] [PubMed] [Google Scholar]
- Langer T., Strober W., Levy R. I. The metabolism of low density lipoprotein in familial type II hyperlipoproteinemia. J Clin Invest. 1972 Jun;51(6):1528–1536. doi: 10.1172/JCI106949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Binding of trifluoperazine to the calcium-dependent activator of cyclic nucleotide phosphodiesterase. Mol Pharmacol. 1977 Jul;13(4):690–697. [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Selective binding of antipsychotics and other psychoactive agents to the calcium-dependent activator of cyclic nucleotide phosphodiesterase. J Pharmacol Exp Ther. 1979 Mar;208(3):454–459. [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Ose T., Berg T., Norum K. R., Ose L. Catabolism of (125-I)low density lipoproteins in isolated rat liver cells. Biochem Biophys Res Commun. 1980 Nov 17;97(1):192–199. doi: 10.1016/s0006-291x(80)80153-7. [DOI] [PubMed] [Google Scholar]
- Redgrave T. G., Roberts D. C., West C. E. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal Biochem. 1975 May 12;65(1-2):42–49. doi: 10.1016/0003-2697(75)90488-1. [DOI] [PubMed] [Google Scholar]
- Shechter I., Fogelman A. M., Haberland M. E., Seager J., Hokom M., Edwards P. A. The metabolism of native and malondialdehyde-altered low density lipoproteins by human monocyte-macrophages. J Lipid Res. 1981 Jan;22(1):63–71. [PubMed] [Google Scholar]
- Tanaka T., Hidaka H. Hydrophobic regions function in calmodulin-enzyme(s) interactions. J Biol Chem. 1980 Dec 10;255(23):11078–11080. [PubMed] [Google Scholar]
- Van Berkel T. J., Kruijt J. K., Van Gent T., Van Tol A. Saturable high affinity binding, uptake and degradation of rat plasma lipoproteins by isolated parenchymal and non-parenchymal cells from rat liver. Biochim Biophys Acta. 1981 Jul 24;665(1):22–33. doi: 10.1016/0005-2760(81)90227-7. [DOI] [PubMed] [Google Scholar]
- Van Berkel T. J., Kruijt J. K., Van den Berg G. B., Koster J. F. Difference in the effect of glucagon and starvation upon L-type pyruvate kinase from rat liver. Eur J Biochem. 1978 Dec;92(2):553–561. doi: 10.1111/j.1432-1033.1978.tb12777.x. [DOI] [PubMed] [Google Scholar]
- Van Tol A., Van 't Hooft F. M., Van Gent T. Discrepancies in the catabolic pathways of rat and human low density lipoproteins as revealed by partial hepatectomy in the rat. Atherosclerosis. 1978 Apr;29(4):449–457. doi: 10.1016/0021-9150(78)90173-9. [DOI] [PubMed] [Google Scholar]
- Wurster N. B., Zilversmit D. B. The role of phagocytosis in the development of atherosclerotic lesions in the rabbit. Atherosclerosis. 1971 Nov-Dec;14(3):309–322. doi: 10.1016/0021-9150(71)90060-8. [DOI] [PubMed] [Google Scholar]
- van Berkel T. J. Difference spectra, catalase- and peroxidase activities of isolated parenchymal and non-parenchymal cells from rat liver. Biochem Biophys Res Commun. 1974 Nov 6;61(1):204–209. doi: 10.1016/0006-291x(74)90553-1. [DOI] [PubMed] [Google Scholar]
- van Berkel T. J., Nagelkerke J. F., Kruijt J. K. The effect of Ca2+ and the trifluoperazine on the processing of human acetylated low density lipoprotein by non-parenchymal liver cells. FEBS Lett. 1981 Sep 14;132(1):61–66. doi: 10.1016/0014-5793(81)80427-9. [DOI] [PubMed] [Google Scholar]
- van Berkel T. J., van Tol A. In vivo uptake of human and rat low density and high density lipoprotein by parenchymal and nonparenchymal cells from rat liver. Biochim Biophys Acta. 1978 Aug 25;530(2):299–304. doi: 10.1016/0005-2760(78)90015-2. [DOI] [PubMed] [Google Scholar]
- van Tol A., van Berkel T. J. Uptake and degradation of rat and human very low density (remnant) apolipoprotein by parenchymal and non-parenchymal rat liver cells. Biochim Biophys Acta. 1980 Jul 14;619(1):156–166. doi: 10.1016/0005-2760(80)90251-9. [DOI] [PubMed] [Google Scholar]


