Abstract
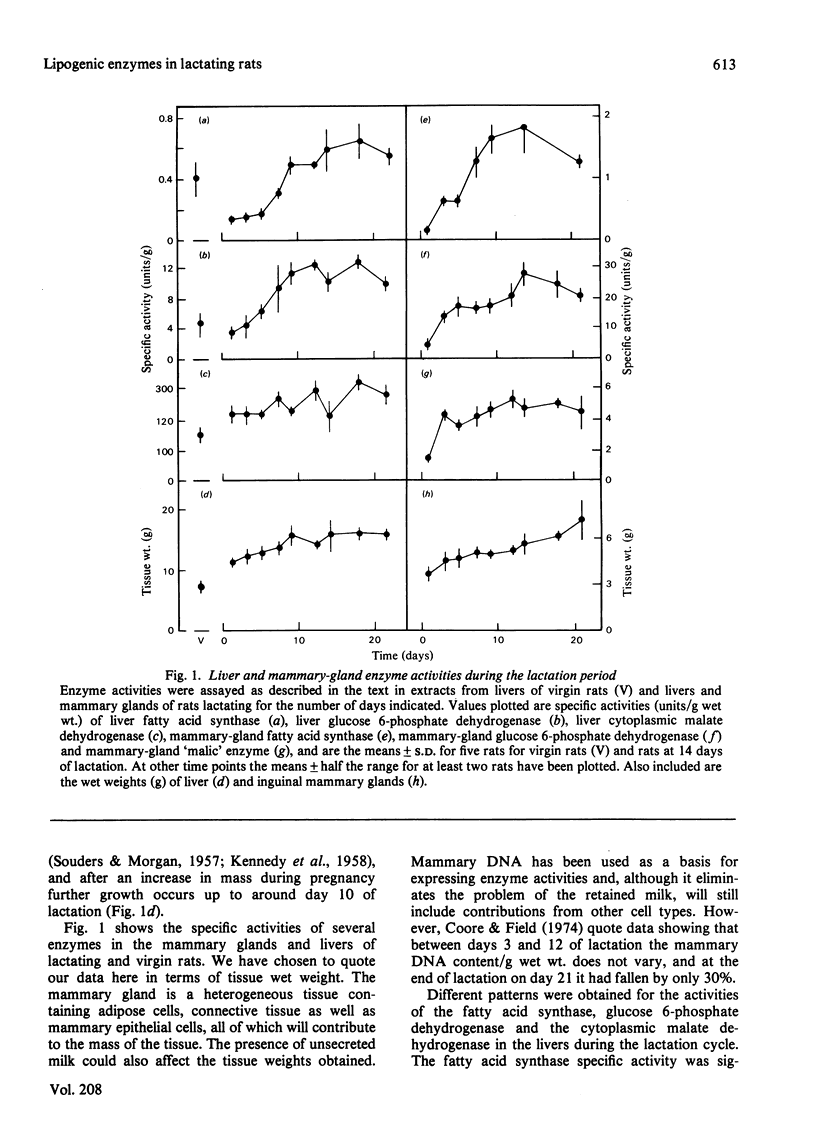
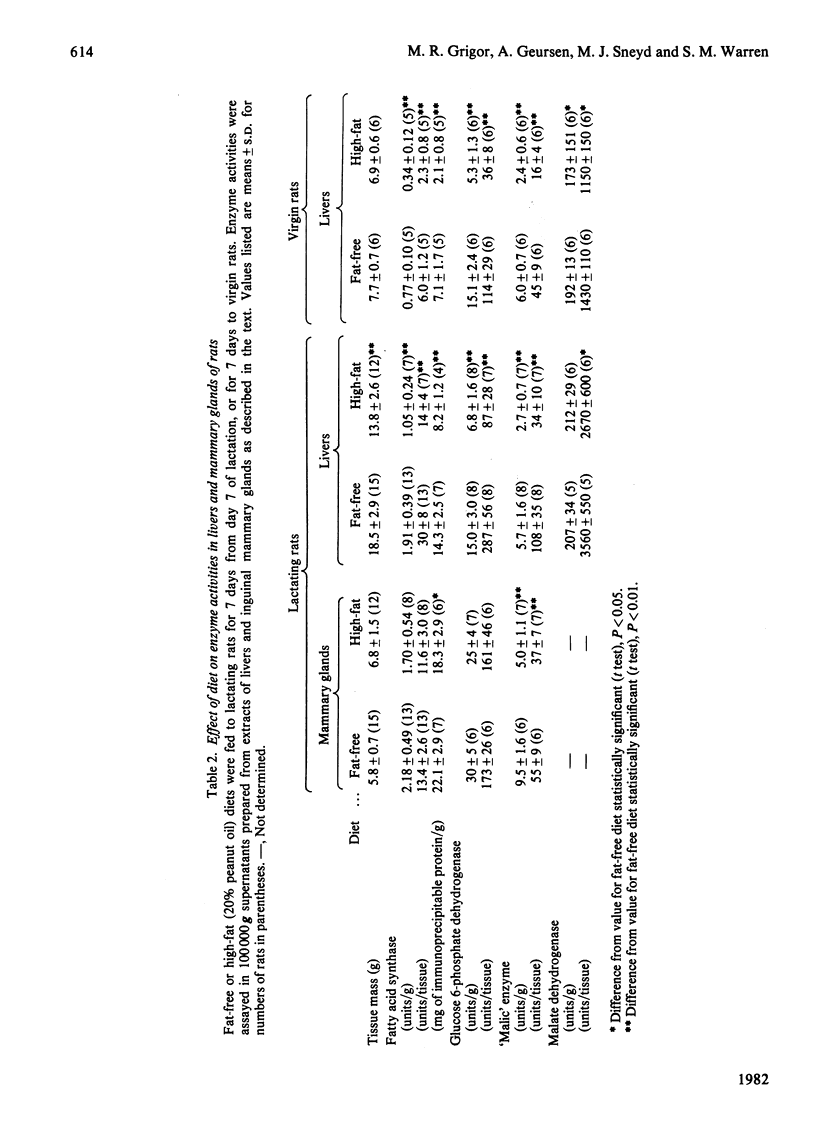
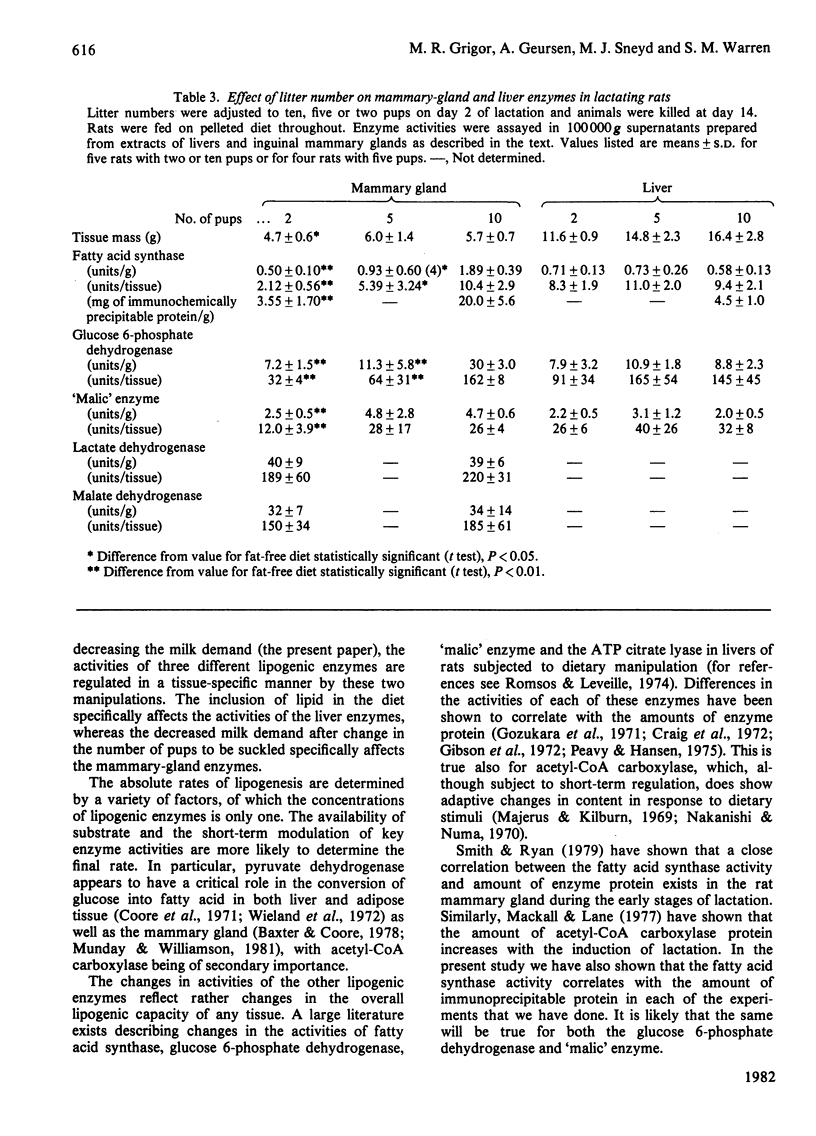
1. The rate of mammary-gland lipogenesis measured in vivo from 3H2O was suppressed after decreasing the milk demand by decreasing the number of pups from ten to two or three, as well as by giving diets containing lipid [Grigor & Warren (1980) Biochem. J. 188, 61-65]. 2. The specific activities of the lipogenic enzymes fatty acid synthase, glucose 6-phosphate dehydrogenase and 'malic' enzyme increased between 6- and 10-fold in the mammary gland and between 2- and 3-fold in the livers during the first 10 days of lactation. The increases in specific activity coupled with the doubling of liver mass which occurred during pregnancy and lactation resulted in considerable differences in total liver activities when compared with virgin animals. 3. Although consumption of a diet containing 20% peanut oil suppressed the activities of the three lipogenic enzymes in the livers, only the 'malic' enzyme was affected in the mammary glands. 4. In contrast, decreased milk demand did not affect the specific activities of any of the liver enzymes, whereas it resulted in suppression of all three lipogenic enzymes of the mammary glands. There was no effect on either the cytoplasmic malate dehydrogenase or the lactate dehydrogenase of the mammary gland. 5. In all the experiments performed, the activity of the fatty acid synthase correlated with the amount of material precipitated by the rabbit antibody raised against rat fatty acid synthase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin R. L., Milligan L. P. Enzymatic changes associated with the initiation and maintenance of lactation in the rat. J Biol Chem. 1966 May 10;241(9):2058–2066. [PubMed] [Google Scholar]
- Baxter M. A., Coore H. G. The mode of regulation of pyruvate dehydrogenase of lactating rat mammary gland. Effects of starvation and insulin. Biochem J. 1978 Aug 15;174(2):553–561. doi: 10.1042/bj1740553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coore H. G., Field B. Properties of pyruvate dehydrogenase of rat mammary tissue and its changes during pregnancy, lactation and weaning. Biochem J. 1974 Jul;142(1):87–95. doi: 10.1042/bj1420087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig M. C., Nepokroeff C. M., Lakshmanan M. R., Porter J. W. Effect of dietary change on the rates of synthesis and degradation of rat liver fatty acid synthetase. Arch Biochem Biophys. 1972 Oct;152(2):619–630. doi: 10.1016/0003-9861(72)90258-5. [DOI] [PubMed] [Google Scholar]
- Garcia D. R., Holten D. Inhibition of rat liver glucose-6-phosphate dehydrogenase synthesis by glucagon. J Biol Chem. 1975 May 25;250(10):3960–3965. [PubMed] [Google Scholar]
- Geisler R. W., Roggeveen A. E., Hansen R. J. The effects of insulin on the turnover of glucose-6-phosphate dehydrogenase in epididymal adipose tissue of the rat. Biochim Biophys Acta. 1978 Dec 1;544(2):284–293. doi: 10.1016/0304-4165(78)90097-1. [DOI] [PubMed] [Google Scholar]
- Gibson D. M., Lyons R. T., Scott D. F., Muto Y. Synthesis and degradation of the lipogenic enzymes of rat liver. Adv Enzyme Regul. 1972;10:187–204. doi: 10.1016/0065-2571(72)90014-3. [DOI] [PubMed] [Google Scholar]
- Gozukara E. M., Frolich M., Holten D. The effect of unsaturated fatty acids on the rate of synthesis of rat liver glucose-6-phosphate dehydrogenase. Biochim Biophys Acta. 1972 Nov 24;286(1):155–163. doi: 10.1016/0304-4165(72)90101-8. [DOI] [PubMed] [Google Scholar]
- Grigor M. R., Warren S. M. Dietary regulation of mammary lipogenesis in lactating rats. Biochem J. 1980 Apr 15;188(1):61–65. doi: 10.1042/bj1880061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gul B., Dils R. Enzymic changes in rabbit and rat mammary gland during the lactation cycle. Biochem J. 1969 Apr;112(3):293–301. doi: 10.1042/bj1120293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumaa K. A., Greenbaum A. L., McLean P. Adaptive changes in satellite systems related to lipogenesis in rat and sheep mammary gland and in adipose tissue. Eur J Biochem. 1973 Apr 2;34(1):188–198. doi: 10.1111/j.1432-1033.1973.tb02745.x. [DOI] [PubMed] [Google Scholar]
- Hizi A., Yagil G. On the mechanism of glucose-6-phosphate dehydrogenase regulation in mouse liver. 3. The rate of enzyme synthesis and degradation. Eur J Biochem. 1974 Jun 1;45(1):211–221. doi: 10.1111/j.1432-1033.1974.tb03545.x. [DOI] [PubMed] [Google Scholar]
- Jones E. A. Changes in the enzyme pattern of the mammary gland of the lactating rat after hypophysectomy and weaning. Biochem J. 1967 May;103(2):420–427. doi: 10.1042/bj1030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNEDY G. C., PEARCE W. M., PARROTT D. M. Liver growth in the lactating rat. J Endocrinol. 1958 Jul;17(2):158–160. doi: 10.1677/joe.0.0170158. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Mackall J. C., Lane M. D. Changes in mammary-gland acetyl-coenzyme A carboxylase associated with lactogenic differentiation. Biochem J. 1977 Mar 15;162(3):635–642. doi: 10.1042/bj1620635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus P. W., Kilburn E. Acetyl coenzyme A carboxylase. The roles of synthesis and degradation in regulation of enzyme levels in rat liver. J Biol Chem. 1969 Nov 25;244(22):6254–6262. [PubMed] [Google Scholar]
- Martyn P., Hansen I. A. Initiation of lipogenic enzyme activities in rat mammary glands. Biochem J. 1981 Jul 15;198(1):187–192. doi: 10.1042/bj1980187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday M. R., Williamson D. H. Role of pyruvate dehydrogenase and insulin in the regulation of lipogenesis in the lactating mammary gland of the rat during the starved-refed transition. Biochem J. 1981 Jun 15;196(3):831–837. doi: 10.1042/bj1960831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S., Numa S. Purification of rat liver acetyl coenzyme A carboxylase and immunochemical studies on its synthesis and degradation. Eur J Biochem. 1970 Sep;16(1):161–173. doi: 10.1111/j.1432-1033.1970.tb01068.x. [DOI] [PubMed] [Google Scholar]
- Peavy D. E., Hansen R. J. Immunological titration of rat liver glucose-6-phosphate dehydrogenase from animals fed high and low carbohydrate diets. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1106–1111. doi: 10.1016/0006-291x(75)90471-4. [DOI] [PubMed] [Google Scholar]
- REES E. D., EVERSOLE A. RAT MAMMARY GLAND METABOLISM RELATIVE TO EPITHELIAL AND CONNECTIVE TISSUE CONTENT. Am J Physiol. 1964 Sep;207:595–600. doi: 10.1152/ajplegacy.1964.207.3.595. [DOI] [PubMed] [Google Scholar]
- Romsos D. R., Leveille G. A. Effect of diet on activity of enzymes involved in fatty acid and cholesterol synthesis. Adv Lipid Res. 1974;12(0):97–146. [PubMed] [Google Scholar]
- Romsos D. R., Muiruri K. L., Lin P. Y., Leveille G. A. Influence of dietary fat, fasting, and acute premature weaning on in vivo rates of fatty acid synthesis in lactating mice. Proc Soc Exp Biol Med. 1978 Nov;159(2):308–312. doi: 10.3181/00379727-159-40338. [DOI] [PubMed] [Google Scholar]
- SOUDERS H. J., MORGAN A. F. Weight and composition of organs during the reproductive cycle in the rat. Am J Physiol. 1957 Oct;191(1):1–7. doi: 10.1152/ajplegacy.1957.191.1.1. [DOI] [PubMed] [Google Scholar]
- Saito T., Tomita K. Two types of soluble malic enzyme in rat tissues. J Biochem. 1973 Apr;73(4):803–810. doi: 10.1093/oxfordjournals.jbchem.a130143. [DOI] [PubMed] [Google Scholar]
- Smith S., Abraham S. Fatty acid synthase from lactating rat mammary gland. Methods Enzymol. 1975;35:65–74. doi: 10.1016/0076-6879(75)35139-2. [DOI] [PubMed] [Google Scholar]
- Smith S., Gagné H. T., Pitelka D. R., Abraham S. The effect of dietary fat on lipogenesis in mammary gland and liver from lactating and virgin mice. Biochem J. 1969 Dec;115(4):807–815. doi: 10.1042/bj1150807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S., Ryan P. Asynchronous appearance of two enzymes concerned with medium chain fatty acid synthesis in developing rat mammary gland. J Biol Chem. 1979 Sep 25;254(18):8932–8936. [PubMed] [Google Scholar]
- Speake B. K., Dils R., Mayer R. J. Regulation of enzyme turnover during tissue differentiation. Interactions of insulin, prolactin and cortisol in controlling the turnover of fatty acid synthetase in rabbit mammary gland in organ culture. Biochem J. 1976 Feb 15;154(2):359–370. doi: 10.1042/bj1540359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G., Convery H. J. Insulin: inducer of glucose 6-phosphate dehydrogenase. Life Sci. 1966 Jun;5(12):1139–1146. doi: 10.1016/0024-3205(66)90098-1. [DOI] [PubMed] [Google Scholar]
- Wieland O. H., Patzelt C., Löffler G. Active and inactive forms of pyruvate dehydrogenase in rat liver. Effect of starvation and refeeding and of insulin treatment on pyruvate-dehydrogenase interconversion. Eur J Biochem. 1972 Apr 11;26(3):426–433. doi: 10.1111/j.1432-1033.1972.tb01783.x. [DOI] [PubMed] [Google Scholar]


