Abstract
Research on neurodegenerative diseases has predominantly focused on high-income countries in the Global North. This Series paper describes the state of biomarker evidence for neurodegeneration in the Global South, including Latin America, Africa, and countries in south, east, and southeast Asia. Latin America shows growth in fluid biomarker and neuroimaging research, with notable advancements in genetics. Research in Africa focuses on genetics and cognition but there is a paucity of data on fluid and neuroimaging biomarkers. South and east Asia, particularly India and China, has achieved substantial progress in plasma, neuroimaging, and genetic studies. However, all three regions face several challenges in the form of a lack of harmonisation, insufficient funding, and few comparative studies both within the Global South, and between the Global North and Global South. Other barriers include scarce infrastructure, lack of knowledge centralisation, genetic and cultural diversity, sociocultural stigmas, and restricted access to tools such as PET scans. However, the diverse ethnic, genetic, economic, and cultural backgrounds in the Global South present unique opportunities for bidirectional learning, underscoring the need for global collaboration to enhance the understanding of dementia and brain health.
Editorial note:
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Introduction
The prevalence of cognitive decline and dementia is increasing globally, but with large heterogeneity across regions.1-6 Approximately 60% of people living with dementia are estimated to be from low-income and middle-income countries in the Global South. This population is projected to increase to 71% by 2050,5 representing an increase of 200%,6 with the greatest increase in eastern sub-Saharan Africa, at 357%.6 Paradoxically, most dementia research is based in the Global North, with no robust characterisations of the diversity and disparity of the populations in the Global South.2,7,8 This imbalance highlights the urgent need for strategies to characterise and care for populations in resource-restricted settings.
Biomarkers are crucial for detecting hallmarks of neurodegeneration longitudinally, assessing disease course, confirming clinical diagnosis, standardising clinical research, and providing biological outcomes for clinical trials.9,10 As disease-modifying treatments emerge, biomarkers will become even more valuable.11 Although fundamental definitions of biomarkers can differ,12 in this Series paper we will use a broad definition: objective measures of typical biological or pathological processes,13 including fluids (plasma and cerebrospinal fluid [CSF]), cognition, neuroimaging, and genetics. These measures constitute crucial components for phenotyping and disease characterisation in the Global South and were studied across neurocognitive disorders, including Alzheimer’s disease, mild cognitive impairment (MCI), vascular dementia, frontotemporal lobar degeneration (FTLD), dementia with Lewy bodies, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis (ALS).
For this Series paper, the term Global South will be used instead of the World Bank classification of countries. Although neither term is perfect because of the grouping of countries with different socioeconomic and health dynamics under the same umbrella, the World Bank classification is more fine grain, which can result in a misleading representation. This detailed economic breakdown overlooks the profound inequalities existing within regions such as Latin America, which, despite having countries such as Chile, a high-income country, is one of the most economically disparate regions globally. This region has risk factors distinct from other high-income countries in the Global North,1-6,8 resulting in less than 1% of its population having access to standard biomarkers of neurodegeneration.10,14-16 Current research underscores distinguishable differences in genetic backgrounds, risk factors, environmental influences, and brain phenotypes between populations in the Global North and Global South,1,3,4,6,8,17-20 highlighting the limitations of universal models in capturing complex brain phenotypes. The understanding of the scientific community is also incomplete because of a lack of validation in diverse and underserved populations and the absence of appropriate cutoff scores21,22 and diversity in clinical trials.9,14,23 This scenario is further complicated by the heterogeneity of comorbidities, such as cardiometabolic syndromes, observed in the Global South.3,24 All these factors call for customised approaches that go beyond universal models to deliver better results.
The National Institute of Aging (NIA) Health Disparities Research Framework25 proposes that environmental, sociocultural, behavioural, and biological factors work together to influence ageing, where race is a sociocultural factor rather than a biological one,3,26-28 emphasising biological and cultural interactions.14,23,29-32 Similarly, allostasis models have evidenced the effect of the physical and social exposomes across pathophysiological pathways.20,30,32-34 This multifactorial framework can be applied to the new definitions proposed by the NIA and the Alzheimer’s Association, contributing to refining the AT(N) binary scheme in Alzheimer’s disease. Systematic inclusion of regions in the Global South in biomarker research offers the best opportunity to examine this framework in a more complex but realistic way.
This Series paper considers representative research conducted on fluid, cognitive, neuroimaging, and genetic biomarkers in Latin America, Africa, and countries in south, east, and southeast Asia (hereafter referred to as South-East Asia), to contextualise biomarker research in the Global South. Rather than a narrow focus, the aim of this selective review is to provide an overview of the state of biomarker research in the Global South, including gaps and potential opportunities. The challenges and unique opportunities for bidirectional learning between the Global South and Global North are then discussed with recommendations for research and policy initiatives to boost biomarker research in the Global South.
Overview of biomarker findings across the Global South
The search and identification strategies are summarised in the appendix p 2. Following screening, 455 studies were included and summarised in tables for the review (table 1). Details of the studies are provided in the appendix pp 5-140. Differences in the areas of focus of the studies included were observed across regions and biomarker types (figure 1). Additional summary information on the studies is included in the appendix p 4.
Table 1:
Overview of the biomarker studies reviewed in this work
| Number of studies | ||||
|---|---|---|---|---|
| Plasma or CSF |
Cognitive | Neuroimaging | Genetics | |
| Latin America | ||||
| Argentina | 7 | 3 | 11 | 9 |
| Brazil | 6 | 2 | 27 | 60 |
| Mexico | 5 | 2 | 1 | 17 |
| Chile | 2 | 2 | ⋅⋅ | 5 |
| Bolivia | ⋅⋅ | ⋅⋅ | ⋅⋅ | ⋅⋅ |
| Peru | 1 | 2 | ⋅⋅ | 1 |
| Colombia | 1 | 1 | 16 | 23 |
| Panama | 1 | ⋅⋅ | ⋅⋅ | 1 |
| Venezuela | ⋅⋅ | ⋅⋅ | ⋅⋅ | 3 |
| Multiple countries in the Caribbean | 1 | ⋅⋅ | 1 | 25 |
| Ecuador | ⋅⋅ | ⋅⋅ | ⋅⋅ | 4 |
| Costa Rica | ⋅⋅ | ⋅⋅ | ⋅⋅ | 1 |
| Total Latin America | 24 | 10* | 58† | 149 |
| Africa | ||||
| Nigeria | ⋅⋅ | 4 | 2 | 4 |
| Tunisia | 1 | ⋅⋅ | ⋅⋅ | 4 |
| Morocco | ⋅⋅ | ⋅⋅ | ⋅⋅ | 2 |
| South Africa | ⋅⋅ | 2 | 1 | 1 |
| Uganda | ⋅⋅ | ⋅⋅ | 1 | ⋅⋅ |
| Tanzania | ⋅⋅ | 9 | ⋅⋅ | ⋅⋅ |
| Kenya | ⋅⋅ | 1 | ⋅⋅ | ⋅⋅ |
| Republic of the Congo | ⋅⋅ | 1 | ⋅⋅ | ⋅⋅ |
| Ghana | ⋅⋅ | 1 | ⋅⋅ | ⋅⋅ |
| Cameroon | ⋅⋅ | 1 | ⋅⋅ | ⋅⋅ |
| Zambia | ⋅⋅ | ⋅⋅ | ⋅⋅ | 1 |
| Democratic Republic of the Congo | 1 | ⋅⋅ | ⋅⋅ | ⋅⋅ |
| Total Africa | 2 | 18* | 4 | 12 |
| South-East Asia | ||||
| India | 13 | 3 | 14 | 17 |
| China | 28 | 1 | 2 | 7 |
| Taiwan | 10 | ⋅⋅ | 4 | ⋅⋅ |
| Thailand | 2 | 1 | 1 | 4 |
| South Korea | 8 | 3 | 23 | 7 |
| Singapore | 10 | 3 | 7 | 3 |
| Indonesia | ⋅⋅ | 1 | ⋅⋅ | ⋅⋅ |
| Philippines | ⋅⋅ | ⋅⋅ | ⋅⋅ | ⋅⋅ |
| Malaysia | ⋅⋅ | 3 | 1 | 4 |
| Sri Lanka | 1 | ⋅⋅ | ⋅⋅ | 1 |
| Multiple countries | ⋅⋅ | ⋅⋅ | ⋅⋅ | 5 |
| Total South-East Asia | 72 | 15 | 52 | 48 |
CSF=cerebrospinal fluid. *Some studies included multiple countries. †In two studies from the Latin American Consortium, individual countries were not described.
Figure 1: Participant distribution in studies included in the selective review per biomarker type across the Global South.
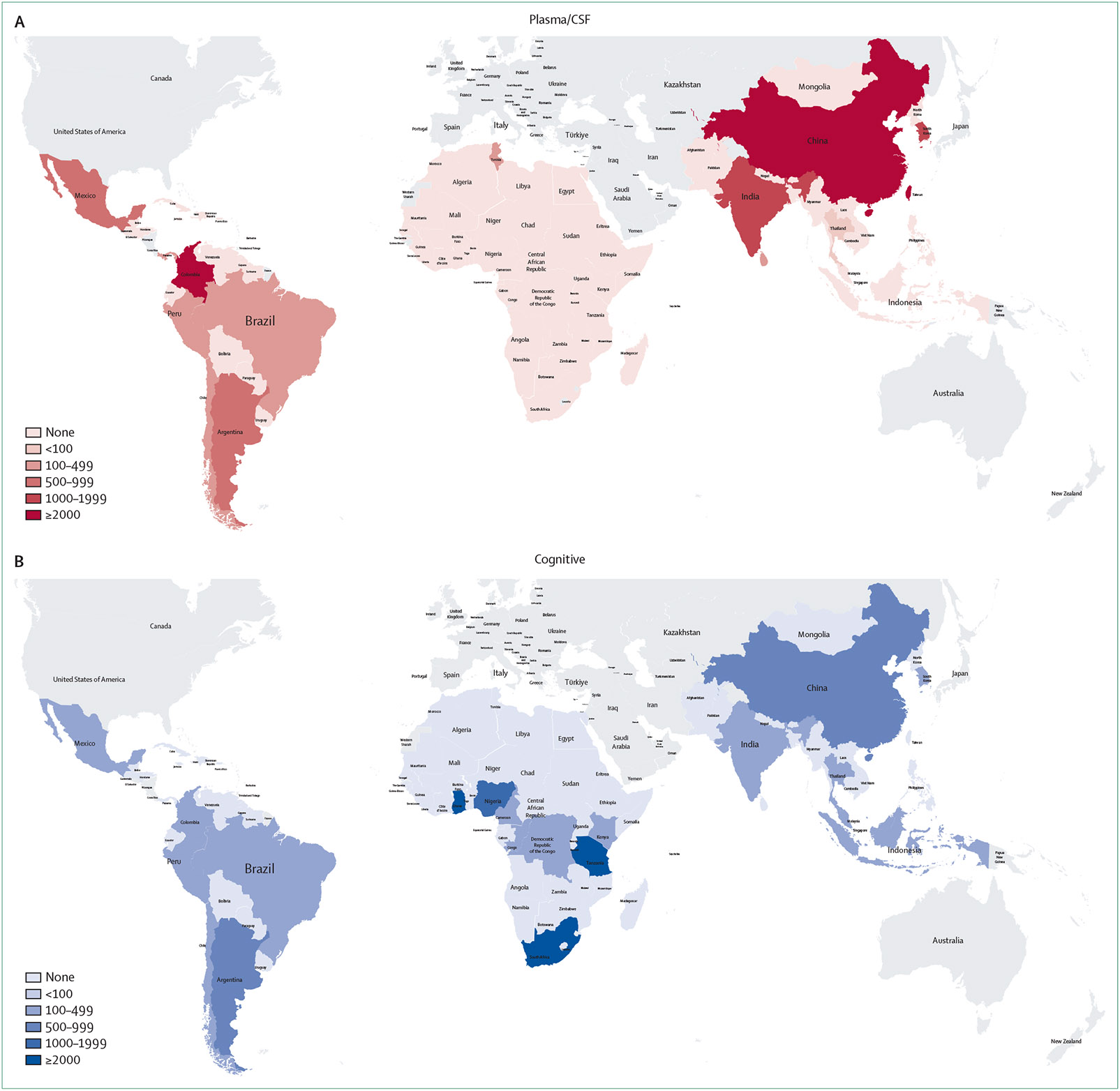
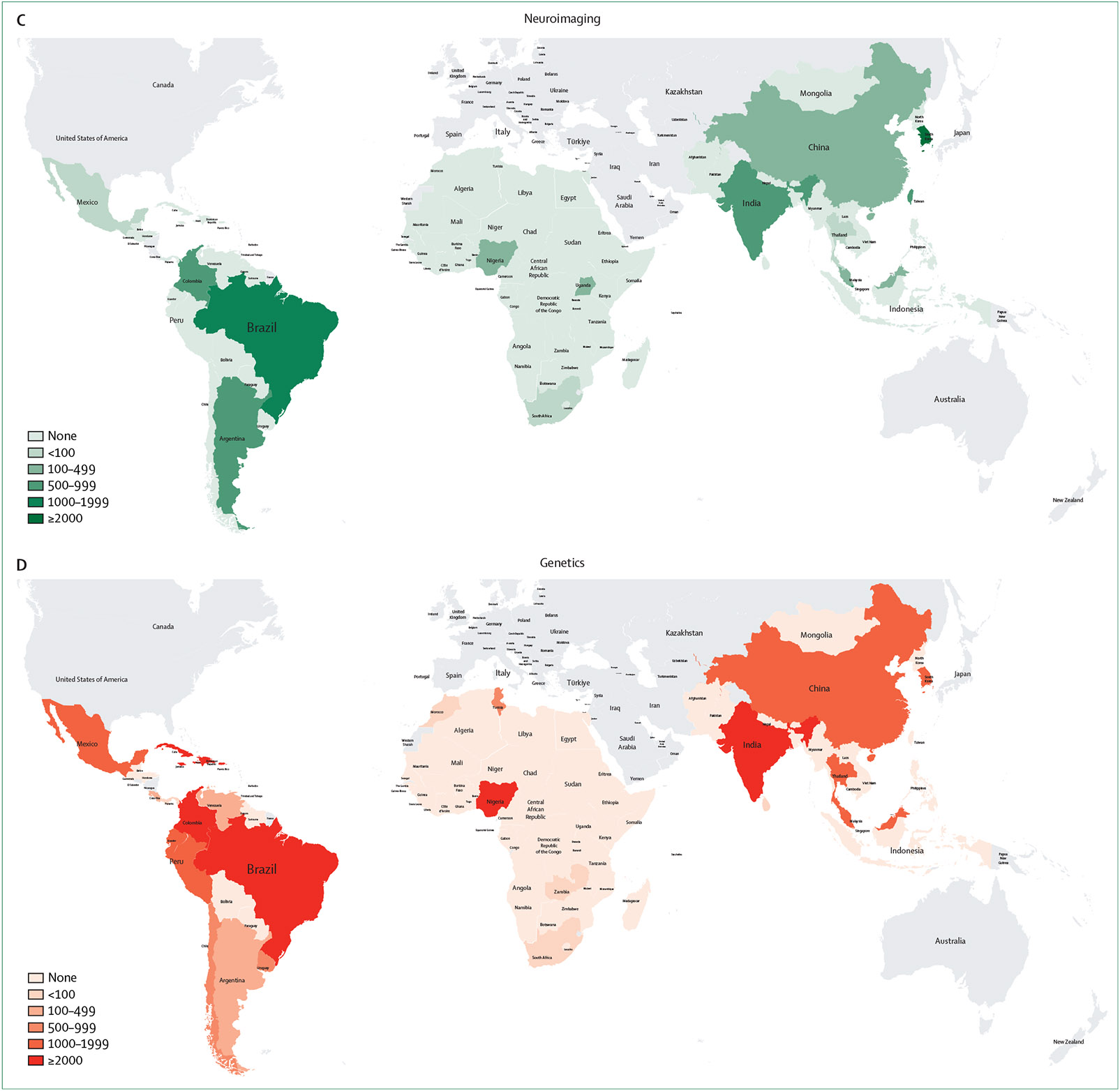
Heatmap illustrating the number of participants included in the selected biomarker studies across countries in the Global South. The figure illustrates the included studies across all types of neurodegenerative diseases across the different biomarker types: fluid biomarkers including plasma and CSF (A), cognitive studies (B), neuroimaging studies (C), and genetic studies (D). Due to the large number of controls in some genetic studies, figures for genetic studies exclude number of controls. CSF=cerebrospinal fluid.
Plasma and CSF
Latin America
The study of fluid biomarkers is progressing in Latin America, with particular focus on Alzheimer’s disease and related dementia. Research in Colombia is focused mostly on carriers of the PSEN1 E280A mutation, related to familial Alzheimer’s disease. Argentina, Brazil, Peru, and Chile are actively involved in CSF biomarker research, although data from several countries in Latin America were notably absent from the reviewed studies. Across studies, classic CSF biomarkers (amyloid β [A β]1–42, total tau, and phosphorylated tau181) consistently differentiated individuals with Alzheimer’s disease from controls.35-38 Individuals with Alzheimer’s disease generally had lower levels of Aβ1–42 and higher levels of total tau and phosphorylated tau181 than did the healthy controls35,39-41 and individuals presenting with MCI;35,39,41-43 in addition, combined biomarker ratios (eg, Aβ1–42 to phosphorylated tau181) showed potential as diagnostic tools. Elevated CSF neurofilament light levels were observed in individuals with Alzheimer’s disease and behavioural variant frontotemporal dementia, compared with those in controls and individuals with MCI.36 With respect to blood (plasma or serum) biomarkers for Alzheimer’s disease, CTACK, MIG, and SDF-1α had discriminative power, with AnxA1 and LX4A capable of distin-guishing Alzheimer’s disease from behavioural variant frontotemporal dementia.37 To the best of our knowledge, no plasma studies with Aβ, tau, or neurodegeneration biomarkers related to sporadic cases of Alzheimer’s disease or FTLD have been performed in Latin America. The incorporation of novel plasma biomarkers (AnxA1, LX4A, and SNCA mRNA) and cytokines showed the potential for expanding research beyond traditional markers.38
Africa
A study related to Alzheimer’s disease carried out in plasma samples from participants from the Democratic Republic of the Congo44 found that plasma Aβ40/42 was significantly associated with lower scores on the Community Screening Interview for Dementia (CSID) and higher scores on the Alzheimer’s Questionnaire in healthy controls, but not in individuals with dementia. However, phosphorylated tau181 displayed no significant associations with either measure in this study. A study in Tunisia that examined CSF biomarkers in 103 participants with Alzheimer’s disease and non-Alzheimer’s disease dementia45 found that the average folate levels in the CSF were lower in participants with Alzheimer’s disease than in controls. No correlation was found between Aβ1–42 or total tau and folate or homocysteine in the CSF. A significant inverse correlation between homocysteine and folate in the CSF was observed in the Alzheimer’s disease group, whereas a significant correlation for homocysteine between the plasma and CSF was found in the non-Alzheimer’s disease group. No other relevant studies were identified.
South-East Asia
India and China were the most active countries in this region for both plasma and CSF research in Alzheimer’s disease and related dementia, with China also having a strong focus on Parkinson’s disease. Taiwan had presence in both plasma and CSF research, whereas Thailand and Singapore primarily focused on CSF biomarker research. Studies from India focused on apolipoprotein E (APOE), interleukin-6, brain-derived neurotrophic factor, clusterin, and others, which identified associations between genetic markers (eg, APOE ε4 allele) and the risk of Alzheimer’s disease or vascular dementia.46-49 Studies in China investigated multiple markers such as Aβ42, total tau, phosphorylated tau, neurofilament light,50-52 and glial fibrillary acidic protein and identified that elevated levels of phosphorylated tau and glial fibrillary acidic protein are associated with cortical thickness.53 Neurofilament light levels were significantly associated with Alzheimer’s disease and inversely correlated with cognitive function. Research carried out in Taiwan examined plasma biomarkers such as Aβ1–42, Aβ1–40, total tau, and phosphorylated tau, with one study highlighting the potential role of SORL1 in Alzheimer’s disease and MCI.54 Although South-East Asia had a larger set of plasma and CSF studies in this review, multiple countries such as Indonesia, the Philippines, Malaysia, and Sri Lanka presented scarce research.
Cognition
Latin America
Most of the research from Latin America originated from countries that have developed multicentric collaboration, such as Argentina, Brazil, Chile, Colombia, Mexico, and Peru. The studies used various cognitive assessments (Mini-Mental State Examination [MMSE], Rowland Universal Dementia Assessment Scale, Montreal Cognitive Assessment [MoCA], Addenbrooke’s Cognitive Examination III, Ineco Frontal Screening, Pfeffer Functional Activity Questionnaire, Neuropsychiatric Inventory, or Mini-Social Cognition & Emotional Assessment) and disease types (Alzheimer’s disease, FTLD, MCI, and Parkinson’s disease), with some studies suggesting that the cognitive assessments allowed for potential disease discrimination.27 Although the level of education of participants27,55 had some influence, these cognitive tests were sensitive and partly specific to disease discrimination. Despite the fact that computational developments have started to control for the substantial sources of variability between studies,27 harmonisation and standardisation of assessments are urgently needed.
Africa
Most research was concentrated in east Africa (Tanzania and Kenya), west Africa (Nigeria), and South Africa, with few reports from north and central Africa. Many African countries were not represented in this research. Studies used various cognitive assessments (Identification of Elderly Africans Instrumental Activities of Daily Living, Alzheimer’s Disease Assessment Scale-Cognitive Subscale, Kiswahili version of the MoCA, India Human Development Survey, or CSID), which include instruments adapted for Africa.56 The CSID, developed by the Ibadan–Indianapolis Dementia Project, was the first key cognitive assessment tool developed for cognitive assessment in Africa.56 The CSID was subsequently translated into multiple local African languages and used for dementia screening in rural populations in Kenya and Tanzania in east Africa. The Identification of Elderly Africans Instrumental Activities of Daily Living Cognitive Screen is a derivative of the CSID, which, at a cutoff of 7 or less, presented sensitivity in the range from 59% for participants in Tanzania57 to 100% for those in Nigeria,58 with the level of education of participants affecting scores.59 In Ibadan, Nigeria, an adequate association was found between the Clinician Home-based Interview to assess Function and Blessed Dementia Scale.60
Adapted cognitive tests such as the Kiswahili version of the MoCA showed acceptable reliability, concurrent validity, sensitivity, and specificity in diagnosing MCI and dementia.61 The strength of these tests flowed from development of culturally adapted cognitive assessments and use of multimodal approaches. However, low representation of African countries in cognitive studies of neurodegeneration underscored the need for further validation in larger and more diverse populations.
South-East Asia
Most studies came from South Korea, Singapore, India, Thailand, Indonesia, and the Philippines using MMSE, MoCA, Visual Cognitive Assessment Test, Boston Naming Test, and Clinical Dementia Rating tools. MMSE, MoCA, and Visual Cognitive Assessment Test were effective in detecting cognitive impairment in Alzheimer’s disease, with MMSE and MoCA useful in detecting cognitive changes in MCI. The Hindi version of MMSE and the Informant Questionnaire on Cognitive Decline in the Elderly were adapted for Hindi-speaking populations, and the Addenbrooke’s Cognitive Examination III was validated in seven Indian languages.62 Cognitive functions in rural Indonesian populations were influenced by the level of education.63 One study combined cognitive assessments with biomarkers and identified significant associations between the Thai version of the Boston Naming Test and the APOE ε4 allele,64 with another study addressing the cultural and educational factors influencing the detection of cognitive impairment using the Hindi version of the MMSE in a comparison between illiterate and literate participants.65 Virtual reality testing also showed potential promise for detecting MCI.66
Neuroimaging
Latin America
A large number of studies included MRI, diffusion tensor imaging, and functional MRI sequences focusing on different aspects, including diagnosis classification, disease stages, and subtype characterisation,67-73 whereas a few studies also conducted PET imaging for assessing brain glucose metabolism, and amyloid and tau load.74-79 The most frequent studies provided associations with clinical and cognitive measures but did not evaluate the association with fluid biomarkers. PET imaging showcased associations between phosphorylated tau217, amplified future amyloid and tau pathologies, and impaired future memory performance.80 Notably, the carriers of the Colombian PSEN1 E280A mutation presented heightened amyloid and tau PET loads.80-82 Distinct correlations were found between sleep patterns, amyloid loads, and cognitive impairments.83 Specific associations between subicular volumes, Aβ deposition, and hippocampal subfield volume reductions emphasised the higher potential of early-phase 11C-Pittsburgh compound B ([11C]PiB)-PET in offering insights into neurodegeneration than [18F]fluorodeoxyglucose ([18F]FDG)-PET.74 Brazilian studies successfully implemented the amyloid and [18F]FDG-PET in the context of the 2018 NIA-Alzheimer’s Association research framework but highlighted clinical biomarker mismatches with sociodemographic effects.
Africa
Overall, studies interrogating neuroimaging markers of neurodegeneration were sparse in Africa, which might be due to the paucity and high cost of relevant neuroimaging modalities. However, a study that evaluated neuroimaging correlates of cognition among survivors of stroke in Nigeria used a range of cognitive measures to map cognitive functionalities against brain measures. The potential vascular basis of neurodegeneration rooted in cerebral hypoperfusion was a relevant insight from that study.84 Additionally, a significant association was reported between carotid artery plaque and abnormal cognitive functions.85 A subsequent study in South Africa conducted analyses of glucose metabolism patterns using [18F]FDG-PET scans to observe association with cognitive imbalances in Parkinson’s disease and parkinsonian-plus disorders, including multiple system atrophy and dementia with Lewy bodies.86 The analyses presented encouraging results regarding sensitivity, specificity, and agreement percentages in diagnoses. A few of the missing focus areas include a substantial gap in the fundamental assessments of neuroimaging for the characterisation of neurodegenerative disease subtypes, stages, and severity. The region had few multicentric studies and comparisons with other regions, and systematically reported publications, indicating a pressing need for a more structured approach to researching neurodegenerative diseases in Africa.
South-East Asia
A comprehensive range of results, primarily from MRI, provided clear delineations about biomarkers between different conditions (Huntington’s disease, MCI, and Alzheimer’s disease) across studies, with correlations with cognition, protein accumulations, and atrophy,87-92 where few assessed functional brain activity.93 Ethnic variations in neuroimaging markers were observed alongside potential markers offering high sensitivity and specificity in detecting cerebrovascular disease and neurodegeneration.90 Multimodal approaches have explored a spectrum of recognised biomarkers, representing a key strength of these studies—the ability to offer a rich and detailed view of the disease processes. However, the vast array of metrics and variables introduced complexity that could overshadow clear conclusions.
Genetics
Latin America
Over 140 genetic studies across Latin America have been conducted, with over 90 focused on Alzheimer’s disease. The rich admixture of Indigenous, African, and European ancestries14-16,70,75 in the region influenced genetic mutations and risk across diseases. For Alzheimer’s disease, the most substantial kindred of PSEN1 was in Colombia, with multiple other genetic presentations of familial Alzheimer’s disease.94-102 Different alleles and mutations in genes such as PSEN1, PSEN2, PICALM, and BIN1, among others, were associated with Alzheimer’s disease to varying degrees.103,104 Strong associations between the APOE ε4 allele and an increased risk of Alzheimer’s disease were accompanied by a protective effect of APOE ε2. Indigenous ancestry and Alzheimer’s disease risk associations suggested some protective benefits,105 whereas African ancestry was associated with increased risk.103 Single-nucleotide polymorphisms and haplotypes in various genes were associated with Alzheimer’s disease risk and protection, with differences noted between Europeans and a Brazilian cohort.104 Although the APOE ε4 is a key risk factor for Alzheimer’s disease in various populations, the role of other genetic factors remains unclear. The detection of G4C2 expansions in the C9orf72 gene was observed in both familial and sporadic FTLD and ALS cases, with a higher prevalence in ALS-FTLD.106-108 Novel mutations and prevalent genetic variations included novel GRN mutations in FTLD cases and significant associations of GRN and MAPT mutations with familial FTLD.109 Genetic variations associated with Parkinson’s disease across ethnic and geographical groups included the recessive model of rs35479735, the variable prevalence of LRRK2 p.G2019S (European ancestry), and risk stratifications of NR4A2, GBA, and MTHFR variations. Other genetic kindreds of additional diseases, such as Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy and Huntington’s disease, were also observed in the region. Leveraging the rich genetic admixture offers unique insights into risk and protective factors.
Africa
Studies identified associations between genetic markers and biomarkers with disease risk and cognitive impairments (Alzheimer’s disease, Parkinson’s disease, ALS, and Huntington’s disease). In Zambia, novel disease-causing variants and deletions have been observed for early-onset Parkinson’s disease.110 A novel genetic risk factor associated with Parkinson’s disease in African and African admixed populations recruited from the International Parkinson’s Disease Genomics Consortium Africa, which has not been seen in the European population, could be a mechanistic basis for Parkinson’s disease in the African population.111 APOE genotypes were associated with cognitive impairment in individuals with Parkinson’s diseas.112 The mutational spectrum in ALS included TARDBP, C9orf72, and SOD1 in Tunisia.113 Carriers of mutations in PSN1 have also been identified. African Americans from Indiana, USA, and the Yoruba population in Nigeria presented links between APOE ε4 homozygosity and increased risk of Alzheimer’s disease and late-onset Alzheimer’s disease; however, this remains a controversial finding in African populations, as the reason for this association remains unclear.114 Protective loci against APOE ε4-associated risk for Alzheimer’s disease in African ancestry populations in Nigeria have also been reported.115 Some participants presented synergistic effects of multiple alleles on dementia risk.116 High prevalence of Huntington’s disease was associated with CAG repeat length in the IT15 gene in a Moroccan population.117 Various African and admixed populations allowed for a rich analysis from diverse geographical and demographic sources, with novel variants identified, in addition to risk and protective factors; however, the small sample sizes in the studies identified limited the robustness of the findings. The cognitive and brain measures used varied substantially across studies, thus making direct comparison of the results challenging.
South-East Asia
A diverse set of insights from research from this region identified well established associations, such as the one between the APOE ε4 allele and Alzheimer’s disease, and novel insights including previously unidentified genetic variations from India that are involved in amyloid signalling.118 Variations and mutations in APOE,119-122 PGRN, MAPT,123,124 and others were associated with disease risk, with strong association between APOE ε4 allele and Alzheimer’s disease and vascular dementia. Conversely, ε3 and ε2 alleles appeared to be protective and ε4 alleles were rare in some cohorts. A small proportion of FTLD cases had GRN mutations.123,125 These include potentially pathogenic gene mutations such as GRN, SQSTM1, LRRK2, NOTCH3, and HTRA1. New MAPT variants increased tau phosphorylation.126 A large study from South Korea identified novel variants of Alzheimer’s disease based on whole-genome sequencing of APOE ε4 carriers.127 Serum brain-derived neurotrophic factor levels were higher in participants with Alzheimer’s disease and amnestic MCI than in controls, although no substantial effects of the Val66Met polymorphism were found on these levels.48 Multiple genetic analysis techniques included whole-genome sequencing, and some studies integrated data types (such as customised Alzheimer’s disease chip data, which focus on specific genetic markers associated with Alzheimer’s disease, and whole-genome sequencing data).127 In Parkinson’s disease, the largest study and a multiethnic cohort on GBA variation from South-East Asia, encompassing Chinese, Malay, and Indian ethnicities, detected three novel variants but no common European risk variants, highlighting the need to include these populations in clinical trials targeting GBA pathways.128
Summary of biomarkers in the Global South
The strengths of the reviewed studies are in the diversity of population sampling, integration of varied data types, and identification of novel genetic variants. Although emerging research provides promising agendas, most of these initiatives were not integrated with one another, did not have multicentric comparisons, and were substantially lower in number, as compared with those carried out in the Global North.1,3,6,8 In Latin America,70,75,129 fluid biomarkers are emerging with a notable focus across the Alzheimer’s disease continuum.129 Cognitive assessments revealed robust disease discrimination, with a need for more harmonised methods. Neuroimaging studies, especially those involving MRI and PET, provided insights into diagnosis, disease stages, and subtype characterisations. Genetic studies offered insights into diverse mutations and risk associations, with the PSEN1 mutation being crucial in Alzheimer’s disease research. In Africa, although the research landscape111 is sparse in terms of plasma and neuroimaging studies, genetic studies presented relevant associations and novel mutations. Cognitive research, largely centred in east and west Africa, emphasised the need for adapted cognitive tests. In South-East Asia, India, China, and Taiwan130,131 conduct most research on plasma and CSF biomarkers in Alzheimer’s disease and Parkinson’s disease, also emphasising the effectiveness of various cognitive tools in detecting impairment. Neuroimaging studies provided a comprehensive understanding of cognitive decline and dementia with genetic research emerging, covering both well established and novel associations. Although not a focus of this study, notably, brain banks have been established in Brazil, Argentina, India, Mexico, China, and Nigeria132,133 (although they are still less common than in the Global North), offering the potential for unique discoveries across neurodegenerative diseases.
Challenges
The Global South is characterised by large heterogeneity within and between regions,1,3,4,8,17-19 with areas of expertise, disparities, and gaps, suggesting the need for different approaches across different regions to support the development of biomarkers. In Latin America and South-East Asia, hubs of expertise in plasma and CSF research are emerging, but plasma research is notably absent in Africa. Evidence of risk associated with the APOE ε4 allele among Africans is conflicting,134 with less than 2% of genome-wide association studies comprising African data.21 As less than 3% of studies originate from African, Indigenous, or Latin American participants,135 little is known about the genetic diversity,14,17,19 polygenic risk scores, socioeconomic disparities,8 or the biology of ageing in these under-represented populations.
At the structural level, funding scarcity, inadequate capacity building, and scarcity of infrastructure create challenges across the regions.10,136-140 PET has been identified as highly limited, prohibitively expensive, and available only in advanced research centres (<1% of the population have access to PET in Latin America).10 Robust computational frameworks to assess multimodal neuroimaging,141 especially new techniques involving whole-brain modeling,20 generative biophysical models,142 and deep learning, are absent.68 Lumbar puncture for CSF collection has been highlighted as a challenge in Brazil, as the technique is available only in large cities and not covered by the public health system.136 Sociocultural stigma is tied to traditional spiritual beliefs, linking behaviours related to dementia to witchcraft in some parts of sub-Saharan Africa,143-145 but without sufficient investigation in understanding how dementia is conceptualised within Indigenous knowledge systems. The introduction of low-cost biomarkers alone might not be sufficient to facilitate dementia diagnosis in the Global South. Although cost is a key factor, logistical, technical, and cultural factors also pose barriers.
A bidirectional approach
A bidirectional approach with the Global North is essential for fostering biomarker research in the Global South, in terms of its goals, methods, benefits, and effects. Limitations of cognitive assessments even within the context of the Global North are acknowledged, with factors such as language of administration, sex or gender, urbanicity, and race or ethnicity possibly affecting cognitive test scores.146 Therefore, although neuropsychological testing is a challenge in the Global South because of the scarcity of standard tools and culturally adapted cutoffs,147 standardisation of instruments might not be sufficient for valid measurement, but might additionally need to be combined with harmonised procedures148 to assess the degree of socioeconomic and cultural heterogeneity. In addition, the limitations and biases of applying methods and findings of the Global North in the context of the Global South should be understood. Risk factor models and brain phenotype associations with stereotypical populations in the Global North do not necessarily apply to the Global South.1-3,16,17,149 Additionally, cardiometabolic factors (such as cardiovascular disease, stroke, and diabetes) that influence some biomarkers affect countries in the Global South3,24 to a substantially greater extent than those in the Global North. Although mortality owing to cardiovascular disease has decreased in the Global North, it has increased in the Global South due to environmental, social, political, and commercial determinants of health.24 Complex interactions between pathophysiological pathways (inflammatory, stress-related, microbiome, and immune) and social and physical exposomes8,33,34,150 represent a challenge for biomarker research. These differences highlight the need to consider adaptations of the Global North strategies for the Global South carefully and the need for capacity building across its regions for localised research, to identify region-specific factors and solutions.
Unique opportunities
The validation of plasma biomarkers of neurodegeneration, including Aβ42/40, phosphorylated tau epitopes, glial fibrillary acidic protein, neurofilament light, and others, in diverse settings will allow for a robust assessment of cognitive health of under-represented populations. Additionally, the bidirectional benefit and effect of advancing biomarker research in the Global South will create opportunities globally. The inclusion of people from regions in the Global South in genomic research on dementia, including genome-wide association study and whole-exome approaches or whole-genome approaches, could provide novel insights into the biology of Alzheimer’s disease and other phenotypes,21 thereby increasing the external validity of results across ethnicities, racial origins, and multicultural backgrounds.151 Such a step will also improve the understanding of polygenic risk factors and interactions with the exposome in the characterisation of biomarkers.
Dementia research at a global level calls for more diversity, where universal and generalisable findings should not be the desired findings across diverse contexts. Insights across brain health, cognitive neuroscience, genetics, and dementia research highlight the inadequacy of universal models for healthy ageing and dementia. Incorporating the multimodal diversity from the Global South can transform the understanding of brain health and dementia globally towards more customised, personalised, and efficient characterisation of biomarkers.
Future prospects
Coordinated efforts across research, policy, and cultural realms (figure 2), including short-term, medium-term, and long-term strategies (table 2), are needed. Although commonalities exist, strategies should be tailored and adapted at the local and regional levels, considering the specific needs of different cultures. Some strategies focus on the development of infrastructure and environment, whereas others focus on ways in which developments in biomarkers could be speedily implemented. For example, the measurement of neurofilament light protein, glial fibrillary acidic protein, phosphorylated tau, and Aβ in dried blood spots152,153 has the advantage of being unaffected by pre-analytical factors, such as time to centrifugation, temperature of freezing, and tube type.
Figure 2: Multisectoral responses to challenges in biomarker research and potential effects in the Global South.
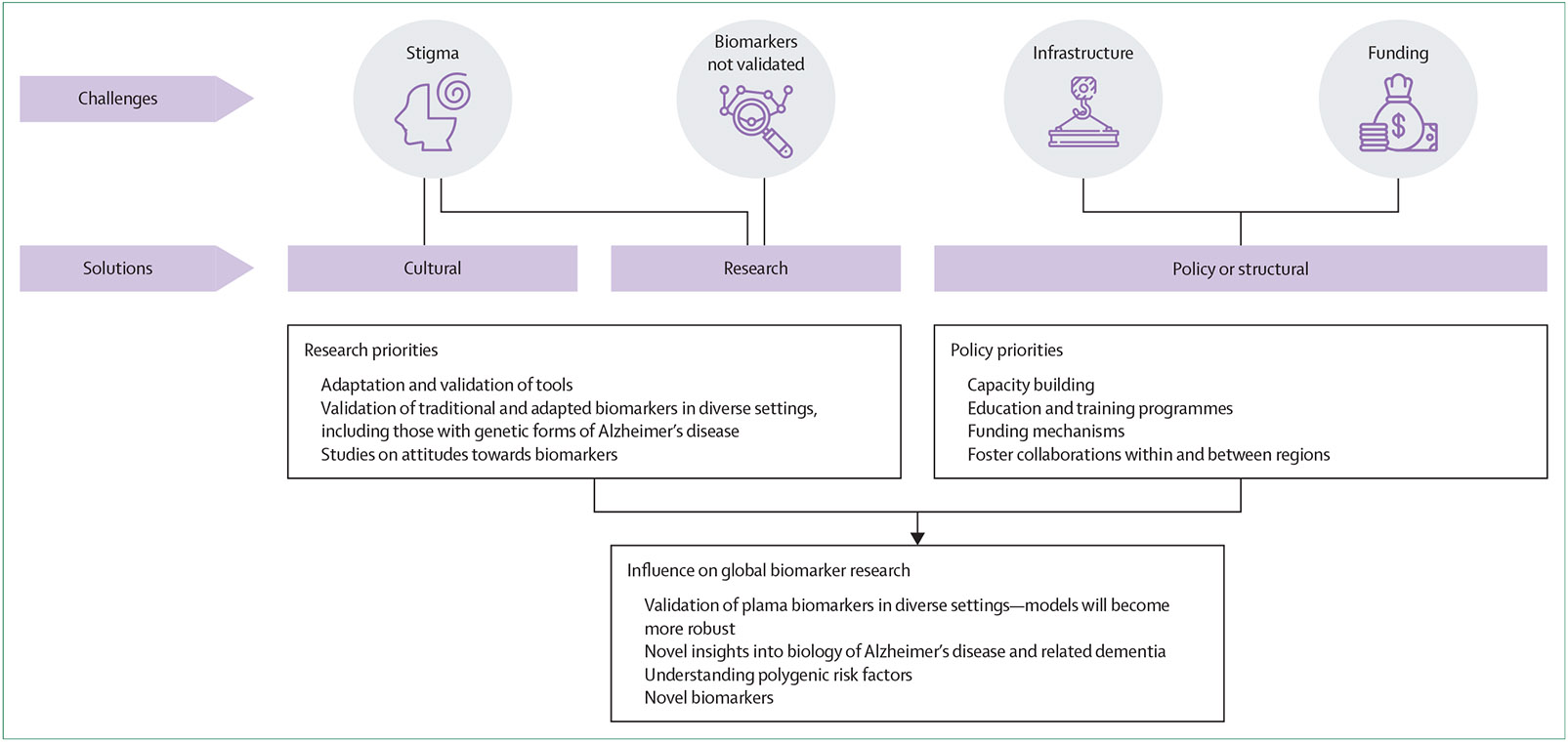
Challenges faced by biomarker research in the Global South and potential solutions are illustrated. Identified challenges such as stigma, validation of biomarkers, infrastructure, and funding require multisectoral solutions across cultural, research, and policy sectors. Suggested research and policy priorities to address these challenges are described. The effects of and potential for biomarker research at a global scale should multiple sectors work together to actualise these priorities are highlighted.
Table 2:
Strategies in different domains for biomarker development across the Global South
| Short-term strategies | Medium-term strategies | Long-term strategies | |
|---|---|---|---|
| Cultural |
|
|
|
| Policy or structural |
|
|
|
| Research |
|
|
|
From a cultural perspective, collaborations with local communities, health-care providers, and policy makers are needed to create a more comprehensive, context-specific approach to mitigate cultural and geographical barriers.138-140,148 Including diverse populations in research on biomarkers should be handled ethically and responsibly, to avoid exploitation. Ethical guidelines and robust community engagement should be integral to this process, with the long-term goal of multisectoral coordination. Global North–Global South partnerships need to redress regional imbalances,154 guided by the principles in documents such as the Africa Charter on Transformative Research Collaborations.155 A pluralistic approach to health care and dementia education and awareness are required to overcome stigma and challenge cultural beliefs of dementia as a prejudiced condition156 and an adscription of witchcraft.144 As a long-term strategy, mobilising resources across regions and disciplines using a brain health diplomacy model156,157 can inform policy around biomarkers and health care.156
One such example of this type of model is the Global Brain Health Institute (GBHI),158 an interdisciplinary training programme based at University of California San Francisco and Trinity College Dublin, which is committed to advancing equity in brain health by training brain health leaders. Similarly, the Alzheimer’s Association supports collaborative research between regions. The largest Latin American consortium on dementia, the Latin American and Caribbean Consortium on Dementia, which includes the Multi-Partner Consortium to Expand Dementia Research in Latin America (ReDLat)15,16,27,68 and the first regional centre (BrainLat),159 was originally supported by GBHI and Alzheimer’s Association. ReDLat studies genetic factors and socioeconomic disparities, such as inequality measures, influencing Alzheimer’s disease and FTLD, involving more than 4000 participants from 13 centres across six countries (Chile, Argentina, Brazil, Peru, Colombia, and Mexico) in Latin America as well as the USA. The study investigates genetic–environmental interactions29,30,160 using harmonised protocols in clinical, cognitive, genomic, and socioeconomic areas across varied Latin American populations. Initially funded in the USA, Argentina, Brazil, Colombia, and Peru, ReDLat later included Mexico and Chile, with additional support from the Alzheimer’s Association, Rainwater Charitable Foundation, and GBHI. Partnerships with National Institutes of Health-Center for Alzheimer’sand Related Dementias, Alector, Takeda Pharmaceuticals, and the Bluefield Foundation have facilitated research on large families with genetic Alzheimer’s disease and FTLD variants. In addition, new National Institutes of Health grants support ecological assessments161 involving epigenomics, speech biomarkers, and circadian imbalances.
In Africa, the African Dementia Consortium brings together over 100 researchers in a multidisciplinary framework to generate clinical and socioeconomic datasets to improve the characterisation of dementia in Africa,162 and the Brain Research Africa Initiative is focused on the translation of brain health evidence for policy and development.163 Each of these approaches illustrates the importance of customised local and regional models that can facilitate translational genomics and improve the understanding of global dementia phenotypes, with potential to identify causal genetic variants.162 The transdisciplinary nature of these stakeholders has the potential for effects across cultural, policy, and research realms to advance research and use of biomarkers, both in the Global South and Global North.
A pressing need for harmonisation across methodologies and regions exists. Establishing a common research agenda on the basis of Global South–Global South and Global South–Global North comparisons could gain richer insights and identify discrepancies. Systematic research protocols would ensure more accurate and reproducible findings, and multicentric, multiregional characterisations would enhance the depth and breadth of studies. Unique regional characteristics, such as the inclusion of Indigenous populations in Latin America, diverse genetic variations highlighted in South-East Asia, and the rich genetic diversity observed in Africa, underscore the need for cross-regional collaborations. Leveraging these unique attributes in a harmonised research framework could accelerate and enhance our understanding of neurodegenerative diseases on a global scale.
Latest advances in research can enhance feasibility in the measurement of neurodegeneration in remote settings, for instance with the use of dried blood spots.152 Similarly, cognitive digital biomarkers in the Global South should be systematically examined to establish their specificity and generalisability across different populations and their potential to be more accessible21 to people in rural areas and outside capital cities.
Conclusions
The biomarker agenda of the Global South needs to overcome both global and region-specific challenges, in addition to recognising its existing strengths and fostering bidirectional collaboration with the Global North. The challenges in infrastructural, financial, technical, cultural, and logistical domains make integrative solutions indispensable. The influence of sociocultural factors on health underscores the importance of localised and context-specific research to suit the uniqueness of different populations, and to better understand the landscape of neurodegeneration in the Global South. However, the silver lining in this landscape is the unique opportunities that emerge from these challenges, with mutual benefits of reducing biases and advancing scientific knowledge at the global level. If approached with an inclusive and ethical mindset, then biomarker research in the Global South can pave the way for groundbreaking discoveries that are globally relevant and locally sensitive. Integration with the multifaceted diversity inherent in these regions will redefine our approaches towards research on brain health, dementia, and biomarkers. For this, the global community should come together to foster collaborations and champion a shared, comprehensive roadmap that is rooted in equity, inclusivity, and innovation.
Supplementary Material
Key messages.
The prevalence of dementia is projected to increase substantially in low-income and middle-income countries in the Global South in the coming years, with an estimated 200% rise by the year 2050, highlighting the urgent need for targeted research and care strategies.
Emerging research on fluid biomarkers in Latin America shows promise in differentiating Alzheimer’s disease cases from controls. Yet, similar studies in Africa are absent, indicating crucial gaps in biomarker research across the Global South.
Cognitive assessments in the Global South indicate robust disease discrimination; however, harmonised methods and culturally adapted tools are needed to improve the accuracy and relevance of patient care across diverse populations.
The Global South faces major challenges in advancing biomarker research in the form of funding scarcity, infrastructure limitations, and sociocultural barriers, necessitating tailored, context-specific strategies and robust community engagement.
Bidirectional collaboration between the Global North and Global South is essential for uncovering new insights into neurodegenerative diseases, improving external validity, and fostering global advancements in biomarker research.
Advancing biomarker research in the Global South offers unique opportunities to validate plasma biomarkers in diverse settings, potentially leading to groundbreaking discoveries in the biology of Alzheimer’s disease and other neurodegenerative conditions.
Coordinated efforts across research, policy, and cultural realms are needed to customise a comprehensive roadmap rooted in equity, inclusivity, and innovation. Concerted efforts should focus on developing infrastructure, implementing culturally sensitive research methods, and ensuring sustainable funding to advance the global understanding and management of neurodegenerative diseases.
Acknowledgments
AI is partly supported by grants from ANID/FONDECYT Regular (1210195, 1210176, and 1220995); ANID/FONDAP/15150012; ANID/PIA/ANILLOS ACT210096; FONDEF ID20I10152; ANID/FONDAP 15150012; and the Multi-Partner Consortium To Expand Dementia Research In Latin America (ReDLat, supported by Fogarty International Center [FIC] and National Institutes of Health, National Institutes of Aging [R01 AG057234, R01 AG075775, R01 AG021051, and CARDS-NIH], Alzheimer’s Association [SG-20-725707], Rainwater Charitable foundation – Tau Consortium, the Bluefield Project to Cure Frontotemporal Dementia, and GBHI). CD-A is partly supported by ANID/FONDECYT Regular 1210622. ERZ is partly supported by CNPq (312410/2018-2, 435642/2018-9, 312306/2021-0, and 409066/2022-2), ARD/FAPERGS (21/2551-0000673-0), Alzheimer’s Association (AARGD-21-850670), CNPQ/FAPERGS/PRONEX (16/2551-0000475-7), the Brazilian National Institute of Science and Technology in Excitotoxicity and Neuroprotection (465671/2014-4), Instituto Serrapilheira (Serra-1912-31365), and Alzheimer’s Association and National Academy of Neuropsychology (ALZ-NAN-22-928381). The contents of this publication are solely the responsibility of the authors and do not represent the official views of these institutions. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Declaration of interests
ERZ is on the scientific advisory board of Nintx; is a co-founder of MASIMA, a Brazilian company that provides brain scan analytical tools to hospitals, is on the scientific advisory board of MASIMA, and has never received royalties of financial gains from MASIMA. All other authors declare no competing interests.
This is the fifth in a Series of six papers about Biomarkers of Neurodegeneration (papers 1, 3, and 4 appear in eBioMedicine). All papers in the Series are available at www.thelancet.com/series/biomarkers-of-neurodegeneration
For more on Criteria for Diagnosis and Staging of Alzheimer’s Disease by Alzheimer’s Association, see https://aaic.alz.org/nia-aa.asp#workgroup
Contributor Information
Eimear McGlinchey, Trinity College Dublin, Dublin, Ireland; Global Brain Health Institute, University of California San Francisco (UCSF), San Francisco, CA, USA; Global Brain Health Institute, Trinity College Dublin, Dublin, Ireland.
Claudia Duran-Aniotz, Latin American Brain Health Institute (BrainLat), Universidad Adolfo Ibanez, Santiago de Chile, Chile.
Rufus Akinyemi, Global Brain Health Institute, University of California San Francisco (UCSF), San Francisco, CA, USA; Global Brain Health Institute, Trinity College Dublin, Dublin, Ireland; Neuroscience and Ageing Research Unit, Institute for Advanced Medical Research and Training; Centre for Genomic and Precision Medicine.
Faheem Arshad, Global Brain Health Institute, University of California San Francisco (UCSF), San Francisco, CA, USA; Global Brain Health Institute, Trinity College Dublin, Dublin, Ireland; College of Medicine, University of Ibadan, Ibadan, Nigeria; National Institute of Mental Health and Neurosciences (NIMHANS), Bengaluru, India.
Eduardo R Zimmer, Department of Pharmacology, Graduate Program in Biological Sciences: Pharmacology and Therapeutics (PPGFT) and Biochemistry (PPGBioq), Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil; Brain Institute of Rio Grande do Sul, Pontificia Universidade Catolica do Rio Grande do Sul, Porto Alegre, Brazil; McGill Centre for Studies in Aging, McGill University, Montreal, QC, Canada.
Hanna Cho, Global Brain Health Institute, University of California San Francisco (UCSF), San Francisco, CA, USA; Global Brain Health Institute, Trinity College Dublin, Dublin, Ireland; Department of Neurology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, South Korea.
Boluwatife Adeleye Adewale, Neuroscience and Ageing Research Unit, Institute for Advanced Medical Research and Training.
Agustin Ibanez, Trinity College Dublin, Dublin, Ireland; Global Brain Health Institute, University of California San Francisco (UCSF), San Francisco, CA, USA; Global Brain Health Institute, Trinity College Dublin, Dublin, Ireland; Latin American Brain Health Institute (BrainLat), Universidad Adolfo Ibanez, Santiago de Chile, Chile.
References
- 1.Walters H. Diverse factors shape healthy aging in Latin America. Nat Aging 2023; 3: 1175. [Google Scholar]
- 2.Ibáñez A, Maito M, Botero-Rodríguez F, et al. Healthy aging metanalyses and scoping review of risk factors across Latin America reveal large heterogeneity and weak predictive models. Nat Aging 2024; published online June 17. 10.1038/s43587-024-00648-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santamaria-Garcia H, Sainz-Ballesteros A, Hernandez H, et al. Factors associated with healthy aging in Latin American populations. Nat Med 2023; 29: 2248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fittipaldi S, Legaz A, Maito M, et al. Heterogeneous factors influence social cognition across diverse settings in brain health and age-related diseases. Nat Ment Health 2024; 2: 63–75. [Google Scholar]
- 5.Guerchet M, Prince M, Prina M. Numbers of people with dementia worldwide: an update to the estimates in the World Alzheimer Report 2015. Nov 30, 2020. https://www.alzint.org/resource/numbers-of-people-with-dementia-worldwide/#:~:text=An%20update%20to%20the%20estimates,low%20and%20middle%20income%20countries (accessed Sept 10, 2023). [Google Scholar]
- 6.GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022; 7: e105–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baez S, Alladi S, Ibanez A. Global South research is critical for understanding brain health, ageing and dementia. Clin Transl Med 2023; 13: e1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ibanez A, Legaz A, Ruiz-Adame M. Addressing the gaps between socioeconomic disparities and biological models of dementia. Brain 2023; 146: 3561–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold M, Amatniek J, Carrillo MC, et al. Digital technologies as biomarkers, clinical outcomes assessment, and recruitment tools in Alzheimer’s disease clinical trials. Alzheimers Dement (N Y) 2018; 4: 234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parra MA, Orellana P, Leon T, et al. Biomarkers for dementia in Latin American countries: gaps and opportunities. Alzheimers Dement 2023; 19: 721–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehrenberg AJ, Khatun A, Coomans E, et al. Relevance of biomarkers across different neurodegenerative diseases. Alzheimers Res Ther 2020; 12: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Califf RM. Biomarker definitions and their applications. Exp Biol Med (Maywood) 2018; 243: 213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Kadmiri N, Said N, Slassi I, El Moutawakil B, Nadifi S. Biomarkers for Alzheimer disease: classical and novel candidates’ review. Neuroscience 2018; 370: 181–90. [DOI] [PubMed] [Google Scholar]
- 14.Parra MA, Baez S, Sedeño L, et al. Dementia in Latin America: paving the way toward a regional action plan. Alzheimers Dement 2021; 17: 295–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ibanez A, Yokoyama JS, Possin KL, et al. The multi-partner consortium to expand dementia research in Latin America (ReDLat): driving multicentric research and implementation science. Front Neurol 2021; 12: 631722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parra MA, Baez S, Allegri R, et al. Dementia in Latin America: assessing the present and envisioning the future. Neurology 2018; 90: 222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greene AS, Shen X, Noble S, et al. Brain–phenotype models fail for individuals who defy sample stereotypes. Nature 2022; 609: 109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020; 396: 413–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fatumo S, Chikowore T, Choudhury A, Ayub M, Martin AR, Kuchenbaecker K. A roadmap to increase diversity in genomic studies. Nat Med 2022; 28: 243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al Zoubi O, Ki Wong C, Kuplicki RT, et al. Predicting age from brain EEG signals-A machine learning approach. Front Aging Neurosci 2018; 10: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akinyemi RO, Yaria J, Ojagbemi A, et al. Dementia in Africa: current evidence, knowledge gaps, and future directions. Alzheimers Dement 2022; 18: 790–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gleason CE, Zuelsdorff M, Gooding DC, et al. Alzheimer’s disease biomarkers in Black and non-Hispanic White cohorts: a contextualized review of the evidence. Alzheimers Dement 2022; 18: 1545–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franzen S, Smith JE, van den Berg E, et al. Diversity in Alzheimer’s disease drug trials: the importance of eligibility criteria. Alzheimers Dement 2022; 18: 810–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miranda JJ, Barrientos-Gutiérrez T, Corvalan C, et al. Understanding the rise of cardiometabolic diseases in low- and middle-income countries. Nat Med 2019; 25: 1667–79. [DOI] [PubMed] [Google Scholar]
- 25.Hill CV, Pérez-Stable EJ, Anderson NA, Bernard MA. The National Institute on Aging Health Disparities Research Framework. Ethn Dis 2015; 25: 245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zugman A, Alliende LM, Medel V, et al. Country-level gender inequality is associated with structural differences in the brains of women and men. Proc Natl Acad Sci U S A 2023; 120: e2218782120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maito MA, Santamaría-García H, Moguilner S, et al. Classification of Alzheimer’s disease and frontotemporal dementia using routine clinical and cognitive measures across multicentric underrepresented samples: a cross sectional observational study. Lancet Reg Health Am 2023; 17: 100387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baez S, Flichtentrei D, Prats M, et al. Men, women…who cares? A population-based study on sex differences and gender roles in empathy and moral cognition. PLoS One 2017; 12: e0179336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Migeot JA, Duran-Aniotz CA, Signorelli CM, Piguet O, Ibáñez A. A predictive coding framework of allostatic-interoceptive overload in frontotemporal dementia. Trends Neurosci 2022; 45: 838–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Felice FG, Gonçalves RA, Ferreira ST. Impaired insulin signalling and allostatic load in Alzheimer disease. Nat Rev Neurosci 2022; 23: 215–30. [DOI] [PubMed] [Google Scholar]
- 31.Bottaccioli AG, Bottaccioli F, Minelli A. Stress and the psyche-brain-immune network in psychiatric diseases based on psychoneuroendocrineimmunology: a concise review. Ann N Y Acad Sci 2019; 1437: 31–42. [DOI] [PubMed] [Google Scholar]
- 32.Gruenewald TL, Seeman TE, Karlamangla AS, Sarkisian CA. Allostatic load and frailty in older adults. J Am Geriatr Soc 2009; 57: 1525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vermeulen R, Schymanski EL, Barabási A-L, Miller GW. The exposome and health: where chemistry meets biology. Science 2020; 367: 392–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ibanez A, Zimmer ER. Time to synergize mental health with brain health. Nat Ment Health 2023; 1: 441–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allegri RF, Chrem Mendez P, Russo MJ, et al. Biomarkers of Alzheimer’s disease in mild cognitive impairment: experience in a memory clinic from Latin America. Neurologia (Engl Ed) 2021; 36: 201–08. [DOI] [PubMed] [Google Scholar]
- 36.Niikado M, Chrem-Méndez P, Itzcovich T, et al. Evaluation of cerebrospinal fluid neurofilament light chain as a routine biomarker in a memory clinic. J Gerontol A Biol Sci Med Sci 2019; 74: 442–45. [DOI] [PubMed] [Google Scholar]
- 37.Gongora-Rivera F, Gonzalez-Aquines A, Ortiz-Jiménez X, de la Garza CM, Salinas-Carmona M. Chemokine profile in Alzheimer’s disease: results from a Mexican population. J Clin Neurosci 2020; 73: 159–61. [DOI] [PubMed] [Google Scholar]
- 38.Fraga VG, Magalhães CA, Loures CMG, et al. Inflammatory and proresolving mediators in frontotemporal dementia and Alzheimer’s disease. Neuroscience 2019; 421: 123–35. [DOI] [PubMed] [Google Scholar]
- 39.Méndez PC, Calandri I, Nahas F, et al. Argentina-Alzheimer’s Disease Neuroimaging Initiative (Arg-ADNI): neuropsychological evolution profile after one-year follow up. Arq Neuropsiquiatr 2018; 76: 231–40. [DOI] [PubMed] [Google Scholar]
- 40.Reis T, Brandão CO, Freire Coutinho ES, Engelhardt E, Laks J. Cerebrospinal fluid biomarkers in Alzheimer’s disease and geriatric depression: preliminary findings from Brazil. CNS Neurosci Ther 2012; 18: 524–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russo MJ, Cohen G, Chrem Mendez P, et al. Predicting episodic memory performance using different biomarkers: results from Argentina-Alzheimer’s Disease Neuroimaging Initiative. Neuropsychiatr Dis Treat 2016; 12: 2199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clarens MF, Crivelli L, Calandri I, et al. Neuropsychological profile of Alzheimer’s disease based on amyloid biomarker findings results from a South American cohort. Appl Neuropsychol Adult 2022; 29: 345–50. [DOI] [PubMed] [Google Scholar]
- 43.Mirandez RM, Aprahamian I, Talib LL, Forlenza OV, Radanovic M. Multiple category verbal fluency in mild cognitive impairment and correlation with CSF biomarkers for Alzheimer’s disease. Int Psychogeriatr 2017; 29: 949–58. [DOI] [PubMed] [Google Scholar]
- 44.Schwinne M, Alonso A, Roberts BR, et al. The association of Alzheimer’s disease-related blood-based biomarkers with cognitive screening test performance in the Congolese population in Kinshasa. J Alzheimers Dis 2024; 97: 1353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smach MA, Edziri H, Charfeddine B, et al. Polymorphism in apoA1 influences high-density lipoprotein cholesterol levels but is not a major risk factor of Alzheimer’s disease. Dement Geriatr Cogn Dis Extra 2011; 1: 249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kandimalla RJ, Prabhakar S, Binukumar Bk, et al. Cerebrospinal fluid profile of amyloid β42 (Aβ42), hTau and ubiquitin in North Indian Alzheimer’s disease patients. Neurosci Lett 2011; 487: 134–38. [DOI] [PubMed] [Google Scholar]
- 47.Mansoori N, Tripathi M, Alam R, et al. IL-6–174 G/C and ApoE gene polymorphisms in Alzheimer’s and vascular dementia patients attending the cognitive disorder clinic of the All India Institute of Medical Sciences, New Delhi. Dement Geriatr Cogn Disord 2010; 30: 461–68. [DOI] [PubMed] [Google Scholar]
- 48.Sonali N, Tripathi M, Sagar R, Vivekanandhan S. Val66Met polymorphism and BDNF levels in Alzheimer’s disease patients in North Indian population. Int J Neurosci 2013; 123: 409–16. [DOI] [PubMed] [Google Scholar]
- 49.Vishnu VY, Modi M, Sharma S, et al. Role of plasma clusterin in Alzheimer’s disease—a pilot study in a tertiary hospital in Northern India. PLoS One 2016; 11: e0166369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li W-W, Shen Y-Y, Tian D-Y, et al. Brain amyloid-β deposition and blood biomarkers in patients with clinically diagnosed Alzheimer’s disease. J Alzheimers Dis 2019; 69: 169–78. [DOI] [PubMed] [Google Scholar]
- 51.Jia JP, Meng R, Sun YX, Sun WJ, Ji XM, Jia LF. Cerebrospinal fluid tau, Aβ1–42 and inflammatory cytokines in patients with Alzheimer’s disease and vascular dementia. Neurosci Lett 2005; 383: 12–16. [DOI] [PubMed] [Google Scholar]
- 52.Wang T, Xiao S, Liu Y, et al. The efficacy of plasma biomarkers in early diagnosis of Alzheimer’s disease. Int J Geriatr Psychiatry 2014; 29: 713–19. [DOI] [PubMed] [Google Scholar]
- 53.Gao F, Dai L, Wang Q, et al. Blood-based biomarkers for Alzheimer’s disease: a multicenter-based cross-sectional and longitudinal study in China. Sci Bull (Beijing) 2023; 68: 1800–08. [DOI] [PubMed] [Google Scholar]
- 54.Chou C-T, Liao Y-C, Lee W-J, Wang S-J, Fuh J-L. SORL1 gene, plasma biomarkers, and the risk of Alzheimer’s disease for the Han Chinese population in Taiwan. Alzheimers Res Ther 2016; 8: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Araujo NB, Nielsen TR, Engedal K, Barca ML, Coutinho ES, Laks J. Diagnosing dementia in lower educated older persons: validation of a Brazilian Portuguese version of the Rowland Universal Dementia Assessment Scale (RUDAS). Braz J Psychiatry 2018; 40: 264–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hall KS, Gao S, Emsley CL, Ogunniyi AO, Morgan O, Hendrie HC. Community screening interview for dementia (CSI ‘D’); performance in five disparate study sites. Int J Geriatr Psychiatry 2000; 15: 521–31. [DOI] [PubMed] [Google Scholar]
- 57.Gray WK, Paddick SM, Collingwood C, et al. Community validation of the IDEA study cognitive screen in rural Tanzania. Int J Geriatr Psychiatry 2016; 31: 1199–207. [DOI] [PubMed] [Google Scholar]
- 58.Paddick S-M, Gray WK, Ogunjimi L, et al. Validation of the Identification and Intervention for Dementia in Elderly Africans (IDEA) cognitive screen in Nigeria and Tanzania. BMC Geriatr 2015; 15: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gray WK, Paddick S-M, Ogunniyi A, et al. Population normative data for three cognitive screening tools for older adults in sub-Saharan Africa. Dement Neuropsychol 2021; 15: 339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hendrie HC, Lane KA, Ogunniyi A, et al. The development of a semi-structured home interview (CHIF) to directly assess function in cognitively impaired elderly people in two cultures. Int Psychogeriatr 2006; 18: 653–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Masika GM, Yu DSF, Li PWC. Accuracy of the Montreal cognitive assessment in detecting mild cognitive impairment and dementia in the rural African population. Arch Clin Neuropsychol 2021; 36: 371–80. [DOI] [PubMed] [Google Scholar]
- 62.Mekala S, Paplikar A, Mioshi E, et al. Dementia diagnosis in seven languages: the Addenbrooke’s Cognitive Examination-III in India. Arch Clin Neuropsychol 2020; 35: 528–38. [DOI] [PubMed] [Google Scholar]
- 63.Sjahrir H, Ritarwan K, Tarigan S, Rambe AS, Lubis ID, Bhakti I. The Mini Mental State Examination in healthy individuals in Medan, Indonesia by age and education level. Neurol J Seast Asia 2001; 6: 19–22. [Google Scholar]
- 64.Aniwattanapong D, Tangwongchai S, Supasitthumrong T, et al. Validation of the Thai version of the short Boston Naming Test (T-BNT) in patients with Alzheimer’s dementia and mild cognitive impairment: clinical and biomarker correlates. Aging Ment Health 2019; 23: 840–50. [DOI] [PubMed] [Google Scholar]
- 65.Tiwari SC, Tripathi RK, Kumar A. Applicability of the Mini-mental State Examination (MMSE) and the Hindi Mental State Examination (HMSE) to the urban elderly in India: a pilot study. Int Psychogeriatr 2009; 21: 123–28. [DOI] [PubMed] [Google Scholar]
- 66.Jang S, Choi SW, Son SJ, et al. Virtual reality-based monitoring test for MCI: a multicenter feasibility study. Front Psychiatry 2022; 13: 1057513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Perl YS, Zamora-Lopez G, Montbrió E, et al. The impact of regional heterogeneity in whole-brain dynamics in the presence of oscillations. Netw Neurosci 2023; 7: 632–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moguilner S, Whelan R, Adams H, Valcour V, Tagliazucchi E, Ibáñez A. Visual deep learning of unprocessed neuroimaging characterises dementia subtypes and generalises across non-stereotypic samples. EBioMedicine 2023; 90: 104540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cruzat J, Herzog R, Prado P, et al. Temporal irreversibility of large-scale brain dynamics in Alzheimer’s disease. J Neurosci 2023; 43: 1643–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moguilner S, Birba A, Fittipaldi S, et al. Multi-feature computational framework for combined signatures of dementia in underrepresented settings. J Neural Eng 2022; 19: 046048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Legaz A, Abrevaya S, Dottori M, et al. Multimodal mechanisms of human socially reinforced learning across neurodegenerative diseases. Brain 2022; 145: 1052–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Herzog R, Rosas FE, Whelan R, et al. Genuine high-order interactions in brain networks and neurodegeneration. Neurobiol Dis 2022; 175: 105918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Birba A, Santamaría-García H, Prado P, et al. Allostatic-interoceptive overload in frontotemporal dementia. Biol Psychiatry 2022; 92: 54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carneiro CG, Faria DP, Coutinho AM, et al. Evaluation of 10-minute post-injection 11C-PiB PET and its correlation with 18F-FDG PET in older adults who are cognitively healthy, mildly impaired, or with probable Alzheimer’s disease. Braz J Psychiatry 2022; 44: 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cecchini MA, Yassuda MS, Squarzoni P, et al. Deficits in short-term memory binding are detectable in individuals with brain amyloid deposition in the absence of overt neurodegeneration in the Alzheimer’s disease continuum. Brain Cogn 2021; 152: 105749. [DOI] [PubMed] [Google Scholar]
- 76.Gaubert S, Raimondo F, Houot M, et al. EEG evidence of compensatory mechanisms in preclinical Alzheimer’s disease. Brain 2019; 142: 2096–112. [DOI] [PubMed] [Google Scholar]
- 77.Parmera JB, Coutinho AM, Aranha MR, et al. FDG-PET patterns predict amyloid deposition and clinical profile in corticobasal syndrome. Mov Disord 2021; 36: 651–61. [DOI] [PubMed] [Google Scholar]
- 78.Rondina JM, Ferreira LK, de Souza Duran FL, et al. Selecting the most relevant brain regions to discriminate Alzheimer’s disease patients from healthy controls using multiple kernel learning: a comparison across functional and structural imaging modalities and atlases. Neuroimage Clin 2017; 17: 628–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sánchez SM, Duarte-Abritta B, Abulafia C, et al. White matter fiber density abnormalities in cognitively normal adults at risk for late-onset Alzheimer’s disease. J Psychiatr Res 2020; 122: 79–87. [DOI] [PubMed] [Google Scholar]
- 80.Aguillon D, Langella S, Chen Y, et al. Plasma p-tau217 predicts in vivo brain pathology and cognition in autosomal dominant Alzheimer’s disease. Alzheimers Dement 2023; 19: 2585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fleisher AS, Chen K, Quiroz YT, et al. Florbetapir PET analysis of amyloid-β deposition in the presenilin 1 E280A autosomal dominant Alzheimer’s disease kindred: a cross-sectional study. Lancet Neurol 2012; 11: 1057–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Quiroz YT, Sperling RA, Norton DJ, et al. Association between amyloid and tau accumulation in young adults with autosomal dominant Alzheimer disease. JAMA Neurol 2018; 75: 548–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Borges CR, Piovezan RD, Poyares DR, et al. Subjective sleep parameters in prodromal Alzheimer’s disease: a case-control study. Braz J Psychiatry 2021; 43: 510–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Akinyemi RO, Firbank M, Ogbole GI, et al. Medial temporal lobe atrophy, white matter hyperintensities and cognitive impairment among Nigerian African stroke survivors. BMC Res Notes 2015; 8: 625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mworozi K, Ameda F, Byanyima RK, Nakasujja N. Carotid artery plaque detected on ultrasound is associated with impaired cognitive state in the elderly: a population-based study in Wakiso district, Uganda. J Clin Neurosci 2019; 68: 194–200. [DOI] [PubMed] [Google Scholar]
- 86.Amod FH, Bhigjee AI, Nyakale N. Utility of 18F FDG-PET in Parkinsonism in an African population. eNeurologicalSci 2022; 27: 100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cho H, Choi JY, Hwang MS, et al. In vivo cortical spreading pattern of tau and amyloid in the Alzheimer disease spectrum. Ann Neurol 2016; 80: 247–58. [DOI] [PubMed] [Google Scholar]
- 88.Suh J, Park YH, Kim HR, et al. The usefulness of visual rating of posterior atrophy in predicting rapid cognitive decline in Alzheimer disease: a preliminary study. Int J Geriatr Psychiatry 2019; 34: 625–32. [DOI] [PubMed] [Google Scholar]
- 89.Hong YJ, Park KW, Kang DY, Lee JH. Prediction of Alzheimer’s pathological changes in subjective cognitive decline using the self-report questionnaire and neuroimaging biomarkers. Dement Neurocogn Disord 2019; 18: 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wong LCK, Wong MYZ, Tan CS, et al. Interethnic differences in neuroimaging markers and cognition in Asians, a population-based study. Sci Rep 2020; 10: 2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chong JR, Ashton NJ, Karikari TK, et al. Plasma P-tau181 to Aβ42 ratio is associated with brain amyloid burden and hippocampal atrophy in an Asian cohort of Alzheimer’s disease patients with concomitant cerebrovascular disease. Alzheimers Dement 2021; 17: 1649–62. [DOI] [PubMed] [Google Scholar]
- 92.Chakraborty S, Mandal S, Kundu S, Sau A. Correlation between Clinical Dementia Rating and brain neuroimaging metrics of Alzheimer’s disease: an observational study from a tertiary care institute of Eastern India. Arch Ment Health 2022; 23: 56–61. [Google Scholar]
- 93.Soman SM, Raghavan S, Rajesh PG, et al. Does resting state functional connectivity differ between mild cognitive impairment and early Alzheimer’s dementia? J Neurol Sci 2020; 418: 117093. [DOI] [PubMed] [Google Scholar]
- 94.Abdala BB, Dos Santos JM, Gonçalves AP, et al. Influence of low frequency PSEN1 variants on familial Alzheimer’s disease risk in Brazil. Neurosci Lett 2017; 653: 341–45. [DOI] [PubMed] [Google Scholar]
- 95.Arango D, Cruts M, Torres O, et al. Systematic genetic study of Alzheimer disease in Latin America: mutation frequencies of the amyloid β precursor protein and presenilin genes in Colombia. Am J Med Genet 2001; 103: 138–43. [DOI] [PubMed] [Google Scholar]
- 96.Dumois-Petersen S, Gallegos-Arreola MP, Magaña-Torres MT, Perea-Díaz FJ, Ringman JM, Figuera LE. Autosomal dominant early onset Alzheimer’s disease in the Mexican state of Jalisco: high frequency of the mutation PSEN1 c. 1292C>A and phenotypic profile of patients. Am J Med Genet C Semin Med Genet 2020; 184: 1023–29. [DOI] [PubMed] [Google Scholar]
- 97.Fox-Fuller JT, Martinez JE, Baena A, et al. Memory for semantically related objects differentiates cognitively unimpaired autosomal dominant mutation carriers from non-carrier family members. J Prev Alzheimers Dis 2023; 10: 322–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Itzcovich T, Chrem-Méndez P, Vázquez S, et al. A novel mutation in PSEN1 (p.T119I) in an Argentine family with early- and late-onset Alzheimer’s disease. Neurobiol Aging 2020; 85: 155.e9–12. [DOI] [PubMed] [Google Scholar]
- 99.Ramirez Aguilar L, Acosta-Uribe J, Giraldo MM, et al. Genetic origin of a large family with a novel PSEN1 mutation (Ile416Thr). Alzheimers Dement 2019; 15: 709–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reiman EM, Quiroz YT, Fleisher AS, et al. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer’s disease in the presenilin 1 E280A kindred: a case-control study. Lancet Neurol 2012; 11: 1048–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Takada LT, Aláez-Verson C, Burgute BD, et al. Discovery and validation of dominantly inherited Alzheimer’s disease mutations in populations from Latin America. Alzheimers Res Ther 2022; 14: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yescas P, Huertas-Vazquez A, Villarreal-Molina MT, et al. Founder effect for the Ala431Glu mutation of the presenilin 1 gene causing early-onset Alzheimer’s disease in Mexican families. Neurogenetics 2006; 7: 195–200. [DOI] [PubMed] [Google Scholar]
- 103.Moreno DJ, Pino S, Ríos Á, et al. Genetic ancestry and susceptibility to late-onset Alzheimer disease (LOAD) in the admixed Colombian population. Alzheimer Dis Assoc Disord 2017; 31: 225–31. [DOI] [PubMed] [Google Scholar]
- 104.Kretzschmar GC, Alencar NM, da Silva SSL, et al. GWAS-Top polymorphisms associated with late-onset Alzheimer disease in Brazil: pointing out possible new culprits among non-coding RNAs. Front Mol Biosci 2021; 8: 632314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Benedet AL, Moraes CF, Camargos EF, et al. Amerindian genetic ancestry protects against Alzheimer’s disease. Dement Geriatr Cogn Disord 2012; 33: 311–17. [DOI] [PubMed] [Google Scholar]
- 106.Miranda CM, Bustamante CML, Herrera CL. Abnormal expansion of C9orf72 gene in familial frontotemporal dementia. Rev Med Chil 2017; 145: 896–900 [in Spanish]. [DOI] [PubMed] [Google Scholar]
- 107.Itzcovich T, Xi Z, Martinetto H, et al. Analysis of C9orf72 in patients with frontotemporal dementia and amyotrophic lateral sclerosis from Argentina. Neurobiol Aging 2016; 40: 192.e13–15. [DOI] [PubMed] [Google Scholar]
- 108.Fernández Suarez M, Surace E, Harris P, et al. C9ORF72 G4C2-repeat expansion and frontotemporal dementia first reported case in Argentina. Neurocase 2016; 22: 281–84. [DOI] [PubMed] [Google Scholar]
- 109.Takada LT, Bahia VS, Guimarães HC, et al. GRN and MAPT mutations in 2 Frontotemporal Dementia Research Centers in Brazil. Alzheimer Dis Assoc Disord 2016; 30: 310–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yonova-Doing E, Atadzhanov M, Quadri M, et al. Analysis of LRRK2, SNCA, Parkin, PINK1, and DJ-1 in Zambian patients with Parkinson’s disease. Parkinsonism Relat Disord 2012; 18: 567–71. [DOI] [PubMed] [Google Scholar]
- 111.Rizig M, Bandres-Ciga S, Makarious MB, et al. Identification of genetic risk loci and causal insights associated with Parkinson’s disease in African and African admixed populations: a genome-wide association study. Lancet Neurol 2023; 22: 1015–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Okubadejo N, Okunoye O, Ojo O, et al. APOE E4 is associated with cognitive decline but not with disease risk or age of onset in Nigerians with Parkinson’s disease. Res Sq 2022. 10.21203/rs.3.rs-1753416/v1 (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kacem I, Sghaier I, Peverelli S, et al. Genotype-phenotype correlation in Tunisian patients with amyotrophic lateral sclerosis. Neurobiol Aging 2022; 120: 27–33. [DOI] [PubMed] [Google Scholar]
- 114.Hendrie HC, Murrell J, Baiyewu O, et al. APOE ε4 and the risk for Alzheimer disease and cognitive decline in African Americans and Yoruba. Int Psychogeriatr 2014; 26: 977–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rajabli F, Beecham GW, Hendrie HC, et al. A locus at 19q13.31 significantly reduces the ApoE ε4 risk for Alzheimer’s disease in African ancestry. PLoS Genet 2022; 18: e1009977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Haithem H, Ons A, Salma N, et al. Association between dementia and vascular disease-associated polymorphisms in a Tunisian population. Int J Neurosci 2018; 128: 32–41. [DOI] [PubMed] [Google Scholar]
- 117.Bouhouche A, Regragui W, Lamghari H, et al. Clinical and genetic data of Huntington disease in Moroccan patients. Afr Health Sci 2015; 15: 1232–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Syama A, Sen S, Kota LN, et al. Mutation burden profile in familial Alzheimer’s disease cases from India. Neurobiol Aging 2018; 64: 158.e7–13. [DOI] [PubMed] [Google Scholar]
- 119.Talwar P, Grover S, Sinha J, et al. Multifactorial analysis of a biomarker pool for Alzheimer disease risk in a north Indian population. Dement Geriatr Cogn Disord 2017; 44: 25–34. [DOI] [PubMed] [Google Scholar]
- 120.Jairani PS, Aswathy PM, Gopala S, Verghese J, Mathuranath PS. Interaction with the MAPT H1H1 genotype increases dementia risk in APOE ε4 carriers in a population of southern India. Dement Geriatr Cogn Disord 2016; 42: 255–64. [DOI] [PubMed] [Google Scholar]
- 121.Luthra K, Tripathi M, Grover R, Dwivedi M, Kumar A, Dey AB. Apolipoprotein E gene polymorphism in Indian patients with Alzheimer’s disease and vascular dementia. Dement Geriatr Cogn Disord 2004; 17: 132–35. [DOI] [PubMed] [Google Scholar]
- 122.Chandak GR, Sridevi MU, Vas CJ, Panikker DM, Singh L. Apolipoprotein E and presenilin-1 allelic variation and Alzheimer’s disease in India. Hum Biol 2002; 74: 683–93. [DOI] [PubMed] [Google Scholar]
- 123.Aswathy PM, Jairani PS, Raghavan SK, et al. Progranulin mutation analysis: identification of one novel mutation in exon 12 associated with frontotemporal dementia. Neurobiol Aging 2016; 39: 218.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Das G, Sadhukhan T, Sadhukhan D, et al. Genetic study on frontotemporal lobar degeneration in India. Parkinsonism Relat Disord 2013; 19: 487–89. [DOI] [PubMed] [Google Scholar]
- 125.Tan YJ, Yong ACW, Foo JN, et al. C9orf72 expansions are the most common cause of genetic frontotemporal dementia in a Southeast Asian cohort. Ann Clin Transl Neurol 2023; 10: 568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cheng HR, Lin RR, Li HL, et al. Identification and functional characterization of novel variants of MAPT and GRN in Chinese patients with frontotemporal dementia. Neurobiol Aging 2023; 123: 233–43. [DOI] [PubMed] [Google Scholar]
- 127.Park JH, Park I, Youm EM, et al. Novel Alzheimer’s disease risk variants identified based on whole-genome sequencing of APOE ε4 carriers. Transl Psychiatry 2021; 11: 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lim JL, Lohmann K, Tan AH, et al. Glucocerebrosidase (GBA) gene variants in a multi-ethnic Asian cohort with Parkinson’s disease: mutational spectrum and clinical features. J Neural Transm (Vienna) 2022; 129: 37–48. [DOI] [PubMed] [Google Scholar]
- 129.Blanco K, Salcidua S, Orellana P, et al. Systematic review: fluid biomarkers and machine learning methods to improve the diagnosis from mild cognitive impairment to Alzheimer’s disease. Alzheimers Res Ther 2023; 15: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang Y, Li Y, Ma L. Recent advances in research on Alzheimer’s disease in China. J Clin Neurosci 2020; 81: 43–46. [DOI] [PubMed] [Google Scholar]
- 131.Huded CB, Bharath S, Chandra SR, Sivakumar PT, Varghese M, Subramanian S. Supportive CSF biomarker evidence to enhance the National Institute on Aging-Alzheimer’s Association criteria for diagnosis of Alzheimer’s type dementia–a study from Southern India. Asian J Psychiatr 2015; 13: 44–47. [DOI] [PubMed] [Google Scholar]
- 132.Klioueva N, Bovenberg J, Huitinga I. Banking brain tissue for research. Handb Clin Neurol 2017; 145: 9–12. [DOI] [PubMed] [Google Scholar]
- 133.Akinyemi RO, Salami A, Akinyemi J, et al. Brain banking in low and middle-income countries: raison d’être for the Ibadan Brain Ageing, Dementia and Neurodegeneration (IBADAN) Brain Bank Project. Brain Res Bull 2019; 145: 136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schlesinger D, Grinberg LT, Alba JG, et al. African ancestry protects against Alzheimer’s disease-related neuropathology. Mol Psychiatry 2013; 18: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Duncan L, Shen H, Gelaye B, et al. Analysis of polygenic risk score usage and performance in diverse human populations. Nat Commun 2019; 10: 3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kerwin D, Abdelnour C, Caramelli P, et al. Alzheimer’s disease diagnosis and management: perspectives from around the world. Alzheimers Dement (Amst) 2022; 14: e12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kandiah N, Choi SH, Hu C-J, Ishii K, Kasuga K, Mok VCT. Current and future trends in biomarkers for the early detection of Alzheimer’s disease in Asia: expert opinion. J Alzheimers Dis Rep 2022; 6: 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Maestre G, Carrillo M, Kalaria R, et al. The Nairobi Declaration-Reducing the burden of dementia in low- and middle-income countries (LMICs): declaration of the 2022 Symposium on Dementia and Brain Aging in LMICs. Alzheimers Dement 2023; 3: 1105–08. [DOI] [PubMed] [Google Scholar]
- 139.Leroi I, Karanja W, Adrion ER, et al. Equity and balance in applied dementia research: a charter of conduct and checklist for global collaborations. Int J Geriatr Psychiatry 2022; 37. [DOI] [PubMed] [Google Scholar]
- 140.Salcher-Konrad M, Naci H, McDaid D, et al. Effectiveness of interventions for dementia in low- and middle-income countries: protocol for a systematic review, pairwise and network meta-analysis. BMJ Open 2019; 9: e027851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ibanez A, Kringelbach M, Deco G. A synergetic turn in cognitive neuroscience of brain diseases. Trends Cognitve Sci 2024; 28: 319–38. [DOI] [PubMed] [Google Scholar]
- 142.Moguilner S, Herzog R, Perl YS, et al. Biophysical models applied to dementia patients reveal links between geographical origin, gender, disease duration, and loss of neural inhibition. Alzheimers Res Ther 2024; 16: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Adebisi AT, Salawu MA. Misconception of dementia-related disorders in Sub-Saharan Africa. Front Neurol 2023; 14: 1148076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brooke J, Ojo O. Contemporary views on dementia as witchcraft in sub-Saharan Africa: a systematic literature review. J Clin Nurs 2020; 29: 20–30. [DOI] [PubMed] [Google Scholar]
- 145.Spittel S, Maier A, Kraus E. Awareness challenges of mental health disorder and dementia facing stigmatisation and discrimination: a systematic literature review from Sub-Sahara Africa. J Glob Health 2019; 9: 020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nichols E, Ng DK, Hayat S, et al. Differences in the measurement of cognition for the assessment of dementia across geographic contexts: recommendations for cross-national research. Alzheimers Dement 2022; 19: 1009–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Magklara E, Stephan BCM, Robinson L. Current approaches to dementia screening and case finding in low- and middle-income countries: research update and recommendations. Int J Geriatr Psychiatry 2019; 34: 3–7. [DOI] [PubMed] [Google Scholar]
- 148.Alladi S, Hachinski V. World dementia: one approach does not fit all. Neurology 2018; 91: 264–70. [DOI] [PubMed] [Google Scholar]
- 149.Stephan BCM, Pakpahan E, Siervo M, et al. Prediction of dementia risk in low-income and middle-income countries (the 10/66 Study): an independent external validation of existing models. Lancet Glob Health 2020; 8: e524–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ibanez A, Melloni L, Świeboda P, et al. Neuroecological links of the exposome and One Health. Neuron 2024; 112: 1905–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lourenco MV, Borelli WV, Duran-Aniotz C, Zimmer ER, de Castro SS. Promoting diversity and overcoming publication barriers in Latin American neuroscience and Alzheimer’s disease research: a call to action. Alzheimers Dement (N Y) 2023; 9: e12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zetterberg H, Schott JM. Blood biomarkers for Alzheimer’s disease and related disorders. Acta Neurol Scand 2022; 146: 51–55. [DOI] [PubMed] [Google Scholar]
- 153.Huber H, Blennow K, Zetterberg H, et al. Biomarkers of Alzheimer’s disease and neurodegeneration in dried blood spots—A new collection method for remote settings. Alzheimers Dement 2024; 20: 2340–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rakotonarivo OS, Andriamihaja OR. Global North-Global South research partnerships are still inequitable. Nat Hum Behav 2023; 7: 2042–43. [DOI] [PubMed] [Google Scholar]
- 155.PARC. Africa Charter for Transformative Research Collaborations. 2023. https://bpb-eu-w2.wpmucdn.com/blogs.bristol.ac.uk/dist/1/627/files/2024/05/Africa-Charter-web-new-cover-52e5a047925be9dc.pdf (accessed March 25, 2023).
- 156.Ibáñez A, Pina-Escudero SD, Possin KL, et al. Dementia caregiving across Latin America and the Caribbean and brain health diplomacy. Lancet Healthy Longev 2021; 2: e222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dawson WD, Bobrow K, Ibanez A, et al. The necessity of diplomacy in brain health. Lancet Neurol 2020; 19: 972–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Smith D. The next generation of leaders advocating for brain health. Lancet Neurol 2018; 17: 29–30. [DOI] [PubMed] [Google Scholar]
- 159.Duran-Aniotz C, Sanhueza J, Grinberg LT, et al. The Latin American Brain Health Institute, a regional initiative to reduce the scale and impact of dementia. Alzheimers Dement 2022; 18: 1696–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Frisoni GB, Altomare D, Thal DR, et al. The probabilistic model of Alzheimer disease: the amyloid hypothesis revised. Nat Rev Neurosci 2022; 23: 53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ibanez A. The mind’s golden cage and cognition in the wild. Trends Cogn Sci 2022; 26: 1031–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Akinyemi RO, Owolabi MO, Okubadejo N, Ogunniyi A, Kalaria RN, African Dementia Consortium. The African Dementia Consortium. Lancet Neurol 2023; 22: 28–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Njamnshi AK, Ngarka L, Njamnshi WY, et al. The Brain Research Africa Initiative (BRAIN). Lancet Neurol 2023; 22: 467–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


