Abstract
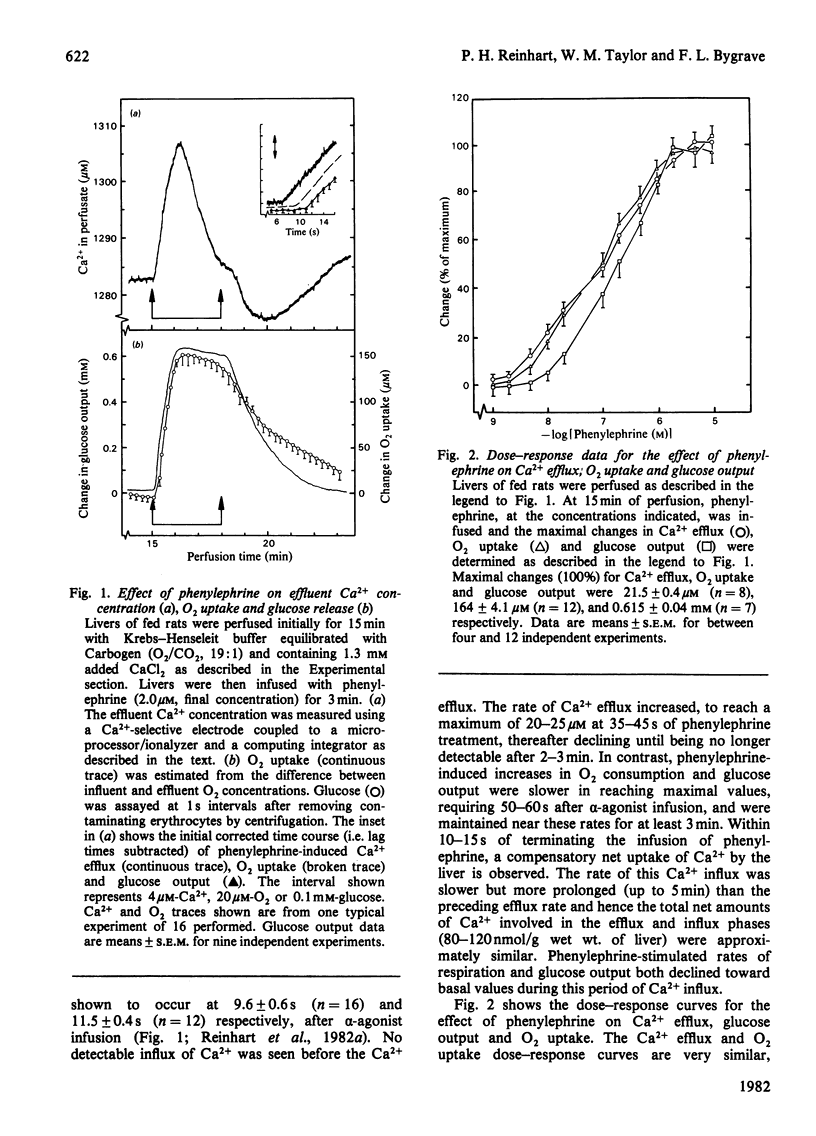
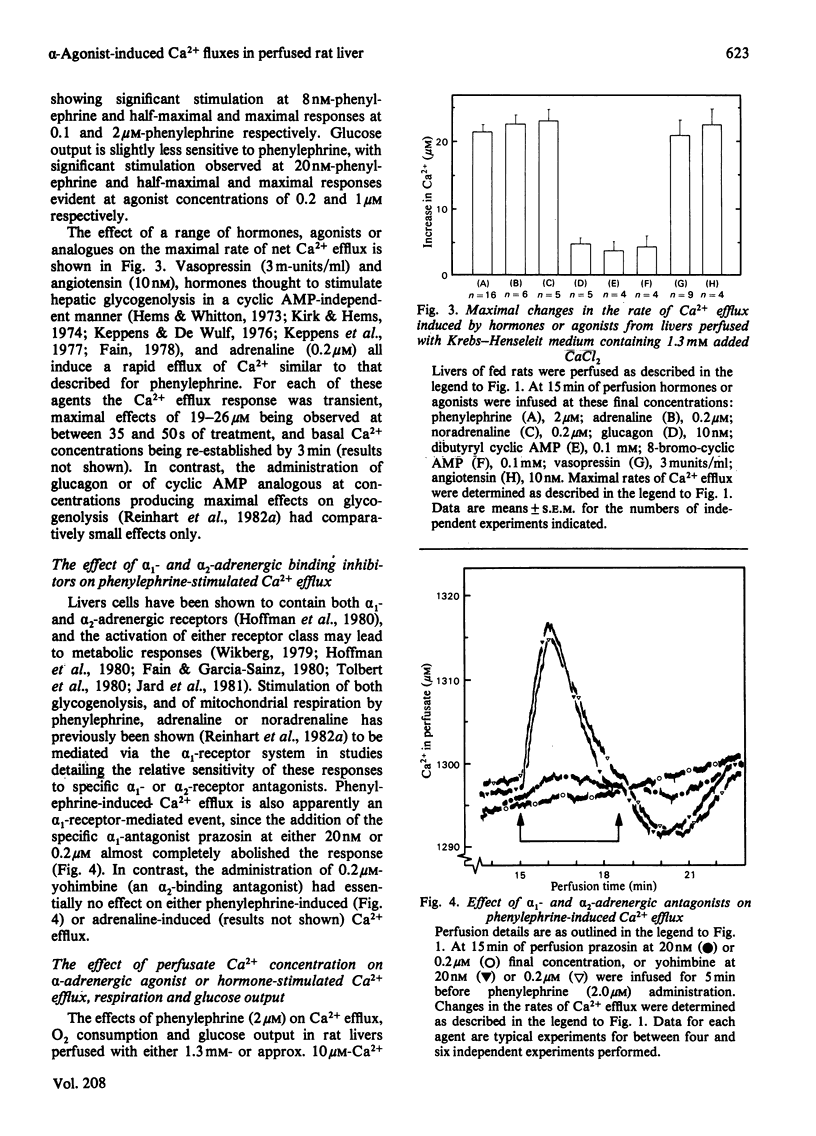
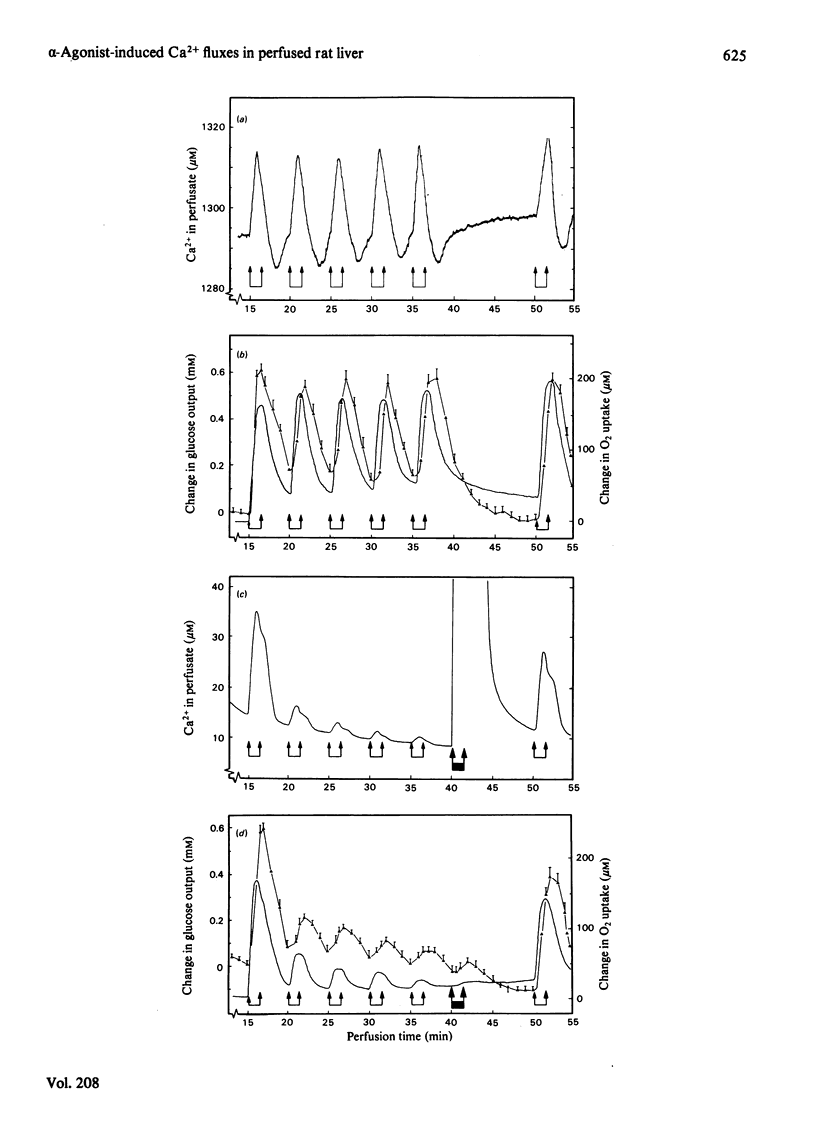
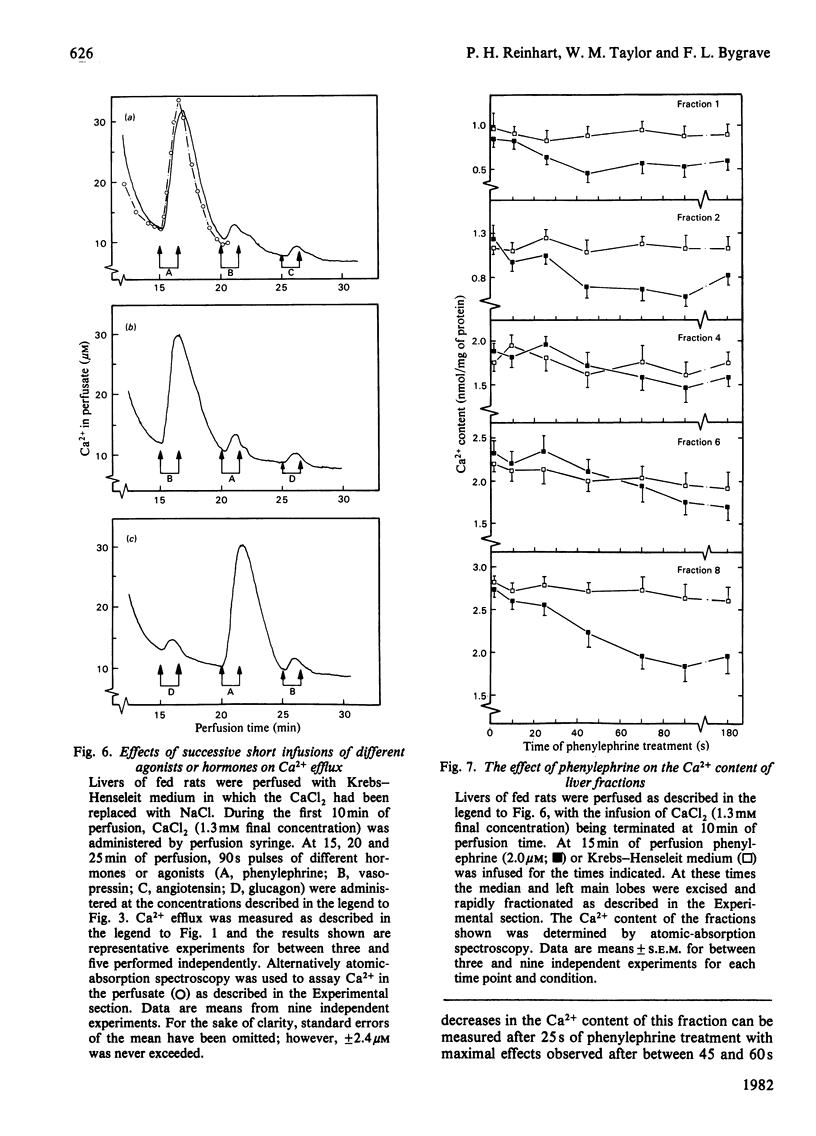
Phenylephrine (2.0 microM) induces an alpha 1-receptor-mediated net efflux of Ca2+ from livers of fed rats perfused with medium containing physiological concentrations (1.3 mM) of Ca2+. The onset of efflux (7.1 +/- 0.5 s; n = 16) immediately precedes a stimulation of mitochondrial respiration and glycogenolysis. Maximal rates of efflux are observed between 35 s and 45 s after alpha-agonist administration; thereafter the rate decreases, to be no longer detectable after 3 min. Within seconds of terminating phenylephrine infusion, a net transient uptake of Ca2+ by the liver is observed. Similar effects were observed with vasopressin (1 m-unit/ml) and angiotensin (6 nM). Reducing the perfusate [Ca2+] from 1.3 mM to 10 microM had little effect on alpha-agonist-induced Ca2+ efflux, but abolished the subsequent Ca2+ re-uptake, and hence led to a net loss of 80-120 nmol of Ca2+/g of liver from the tissue. The administration at 5 min intervals of short pulses (90 s) of phenylephrine under these conditions resulted in diminishing amounts of Ca2+ efflux being detected, and these could be correlated with decreased rates of alpha-agonist-induced mitochondrial respiration and glucose output. An examination of the Ca2+ pool mobilized by alpha-adrenergic agonists revealed that a loss of Ca2+ from mitochondria and from a fraction enriched in microsomes accounts for all the Ca2+ efflux detected. It is proposed that the alpha-adrenergic agonists, vasopressin and angiotensin mobilize Ca2+ from the same readily depleted intracellular pool consisting predominantly of mitochondria and the endoplasmic reticulum, and that the hormone-induced enhanced rate of mitochondrial respiration and glycogenolysis is directly dependent on this mobilization.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Althaus-Salzmann M., Carafoli E., Jakob A. Ca2+, K+ redistributions and alpha-adrenergic activation of glycogenolysis in perfused rat livers. Eur J Biochem. 1980 May;106(1):241–248. doi: 10.1111/j.1432-1033.1980.tb06015.x. [DOI] [PubMed] [Google Scholar]
- Assimacopoulos-Jeannet F. D., Blackmore P. F., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. Studies on role of calcium in alpha-adrenergic activation of phosphorylase. J Biol Chem. 1977 Apr 25;252(8):2662–2669. [PubMed] [Google Scholar]
- Babcock D. F., Chen J. L., Yip B. P., Lardy H. A. Evidence for mitochondrial localization of the hormone-responsive pool of Ca2+ in isolated hepatocytes. J Biol Chem. 1979 Sep 10;254(17):8117–8120. [PubMed] [Google Scholar]
- Barritt G. J., Dalton K. A., Whiting J. A. Evidence that phosphatidic acid stimulates the uptake of calcium by liver cells but not calcium release from mitochondria. FEBS Lett. 1981 Mar 23;125(2):137–140. doi: 10.1016/0014-5793(81)80703-x. [DOI] [PubMed] [Google Scholar]
- Berthon B., Poggioli J., Capiod T., Claret M. Effect of the alpha-agonist noradrenaline on total and 45Ca2+ movements in mitochondria of rat liver cells. Biochem J. 1981 Oct 15;200(1):177–180. doi: 10.1042/bj2000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum M. J., Fain J. N. Activation of protein kinase and glycogen phosphorylase in isolated rat liver cells by glucagon and catecholamines. J Biol Chem. 1977 Jan 25;252(2):528–535. [PubMed] [Google Scholar]
- Blackmore P. F., Brumley F. T., Marks J. L., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. Relationship between alpha-adrenergic stimulation of calcium efflux and activation of phosphorylase in isolated rat liver parenchymal cells. J Biol Chem. 1978 Jul 25;253(14):4851–4858. [PubMed] [Google Scholar]
- Blackmore P. F., Dehaye J. P., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. The role of mitochondrial calcium release in alpha-adrenergic activation of phosphorylase in perfused rat liver. J Biol Chem. 1979 Aug 10;254(15):6945–6950. [PubMed] [Google Scholar]
- Blackmore P. F., Dehaye J. P., Strickland W. G., Exton J. H. alpha-Adrenergic mobilization of hepatic mitochondrial calcium. FEBS Lett. 1979 Apr 1;100(1):117–120. doi: 10.1016/0014-5793(79)81144-8. [DOI] [PubMed] [Google Scholar]
- Blackmore P. F., Hughes B. P., Shuman E. A., Exton J. H. alpha-Adrenergic activation of phosphorylase in liver cells involves mobilization of intracellular calcium without influx of extracellular calcium. J Biol Chem. 1982 Jan 10;257(1):190–197. [PubMed] [Google Scholar]
- Blair J. B., James M. E., Foster J. L. Adrenergic control of glucose output and adenosine 3':5'-monophosphate levels in hepatocytes from juvenile and adult rats. J Biol Chem. 1979 Aug 25;254(16):7579–7584. [PubMed] [Google Scholar]
- Chan T. M., Exton J. H. alpha-Adrenergic-mediated accumulation of adenosine 3':5' monophosphate in calcium-depleted hepatocytes. J Biol Chem. 1977 Dec 10;252(23):8645–8651. [PubMed] [Google Scholar]
- Chen J. L., Babcock D. F., Lardy H. A. Norepinephrine, vasopressin, glucagon, and A23187 induce efflux of calcium from an exchangeable pool in isolated rat hepatocytes. Proc Natl Acad Sci U S A. 1978 May;75(5):2234–2238. doi: 10.1073/pnas.75.5.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrington A. D., Assimacopoulos F. D., Harper S. C., Corbin J. D., Park C. R., Exton J. H. Studies on the alpha-andrenergic activation of hepatic glucose output. II. Investigation of the roles of adenosine 3':5'-monophosphate and adenosine 3':5'-monophosphate-dependent protein kinase in the actions of phenylephrine in isolated hepatocytes. J Biol Chem. 1976 Sep 10;251(17):5209–5218. [PubMed] [Google Scholar]
- Claret-Berthon B., Claret M., Mazet J. L. Fluxes and distribution of calcium in rat liver cells: kinetic analysis and identification of pools. J Physiol. 1977 Nov;272(3):529–552. doi: 10.1113/jphysiol.1977.sp012058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain J. N., García-Sáinz J. A. Role of phosphatidylinositol turnover in alpha 1 and of adenylate cyclase inhibition in alpha 2 effects of catecholamines. Life Sci. 1980 Apr 14;26(15):1183–1194. doi: 10.1016/0024-3205(80)90062-4. [DOI] [PubMed] [Google Scholar]
- Foden S., Randle P. J. Calcium metabolism in rat hepatocytes. Biochem J. 1978 Mar 15;170(3):615–625. doi: 10.1042/bj1700615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems D. A., Whitton P. D. Stimulation by vasopressin of glycogen breakdown and gluconeogenesis in the perfused rat liver. Biochem J. 1973 Nov;136(3):705–709. doi: 10.1042/bj1360705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman B. B., Michel T., Kilpatrick D. M., Lefkowitz R. J., Tolbert M. E., Gilman H., Fain J. N. Agonist versus antagonist binding to alpha-adrenergic receptors. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4569–4573. doi: 10.1073/pnas.77.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson N. J., Brumley F. T., Assimacopoulos F. D., Harper S. C., Exton J. H. Studies on the alpha-adrenergic activation of hepatic glucose output. I. Studies on the alpha-adrenergic activation of phosphorylase and gluconeogenesis and inactivation of glycogen synthase in isolated rat liver parenchymal cells. J Biol Chem. 1976 Sep 10;251(17):5200–5208. [PubMed] [Google Scholar]
- Jard S., Cantau B., Jakobs K. H. Angiotensin II and alpha-adrenergic agonists inhibit rat liver adenylate cyclase. J Biol Chem. 1981 Mar 25;256(6):2603–2606. [PubMed] [Google Scholar]
- Keppens S., De Wulf H. The activation of liver glycogen phosphorylase by angiotensin II. FEBS Lett. 1976 Oct 1;68(2):279–282. doi: 10.1016/0014-5793(76)80453-x. [DOI] [PubMed] [Google Scholar]
- Keppens S., Vandenheede J. R., De Wulf H. On the role of calcium as second messenger in liver for the hormonally induced activation of glycogen phosphorylase. Biochim Biophys Acta. 1977 Feb 28;496(2):448–457. doi: 10.1016/0304-4165(77)90327-0. [DOI] [PubMed] [Google Scholar]
- Kirk C. J., Hems D. A. Hepatic action of vasopressin: lack of a role for adenosine-3',5'-cyclic monophosphate. FEBS Lett. 1974 Oct 1;47(1):128–131. doi: 10.1016/0014-5793(74)80441-2. [DOI] [PubMed] [Google Scholar]
- Lotersztajn S., Hanoune J., Pecker F. A high affinity calcium-stimulated magnesium-dependent ATPase in rat liver plasma membranes. Dependence of an endogenous protein activator distinct from calmodulin. J Biol Chem. 1981 Nov 10;256(21):11209–11215. [PubMed] [Google Scholar]
- Madeira V. M. A rapid and ultrasensitive method to measure Ca++ movements across biological membranes. Biochem Biophys Res Commun. 1975 Jan 2;64(3):870–876. doi: 10.1016/0006-291x(75)90128-x. [DOI] [PubMed] [Google Scholar]
- Murphy E., Coll K., Rich T. L., Williamson J. R. Hormonal effects on calcium homeostasis in isolated hepatocytes. J Biol Chem. 1980 Jul 25;255(14):6600–6608. [PubMed] [Google Scholar]
- Poggioli J., Berthon B., Claret M. Calcium movements in in situ mitochondria following activation of alpha-adrenergic receptors in rat liver cells. FEBS Lett. 1980 Jun 30;115(2):243–246. doi: 10.1016/0014-5793(80)81178-1. [DOI] [PubMed] [Google Scholar]
- Prpić V., Spencer T. L., Bygrave F. L. Stable enhancement of calcium retention in mitochondria isolated from rat liver after the administration of glucagon to the intact animal. Biochem J. 1978 Dec 15;176(3):705–714. doi: 10.1042/bj1760705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart P. H., Bygrave F. L. Glucagon stimulation of ruthenium red-insensitive calcium ion transport in developing rat liver. Biochem J. 1981 Feb 15;194(2):541–549. doi: 10.1042/bj1940541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. A procedure for the rapid preparation of mitochondria from rat liver. Biochem J. 1982 Jun 15;204(3):731–735. doi: 10.1042/bj2040731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. Studies on alpha-adrenergic-induced respiration and glycogenolysis in perfused rat liver. J Biol Chem. 1982 Feb 25;257(4):1906–1912. [PubMed] [Google Scholar]
- Sherline P., Lynch A., Glinsmann W. H. Cyclic AMP and adrenergic receptor control of rat liver glycogen metabolism. Endocrinology. 1972 Sep;91(3):680–690. doi: 10.1210/endo-91-3-680. [DOI] [PubMed] [Google Scholar]
- Taylor W. M., Reinhart P., Hunt N. H., Bygrave F. L. Role of 3',5'-cyclic AMP in glucagon-induced stimulation of ruthenium red-insensitive calcium transport in an endoplasmic reticulum-rich fraction of rat liver. FEBS Lett. 1980 Mar 24;112(1):92–96. doi: 10.1016/0014-5793(80)80136-0. [DOI] [PubMed] [Google Scholar]
- Tolbert M. E., Butcher F. R., Fain J. N. Lack of correlation between catecholamine effects on cyclic adenosine 3':5'-monophosphate and gluconeogenesis in isolated rat liver cells. J Biol Chem. 1973 Aug 25;248(16):5686–5692. [PubMed] [Google Scholar]
- Tolbert M. E., White A. C., Aspry K., Cutts J., Fain J. N. Stimulation by vasopressin and alpha-catecholamines of phosphatidylinositol formation in isolated rat liver parenchymal cells. J Biol Chem. 1980 Mar 10;255(5):1938–1944. [PubMed] [Google Scholar]
- Wikberg J. E. The pharmacological classification of adrenergic alpha 1 and alpha 2 receptors and their mechanisms of action. Acta Physiol Scand Suppl. 1979;468:1–99. [PubMed] [Google Scholar]
- van de Werve G., Hue L., Hers H. G. Hormonal and ionic control of the glycogenolytic cascade in rat liver. Biochem J. 1977 Jan 15;162(1):135–142. doi: 10.1042/bj1620135. [DOI] [PMC free article] [PubMed] [Google Scholar]


