Abstract
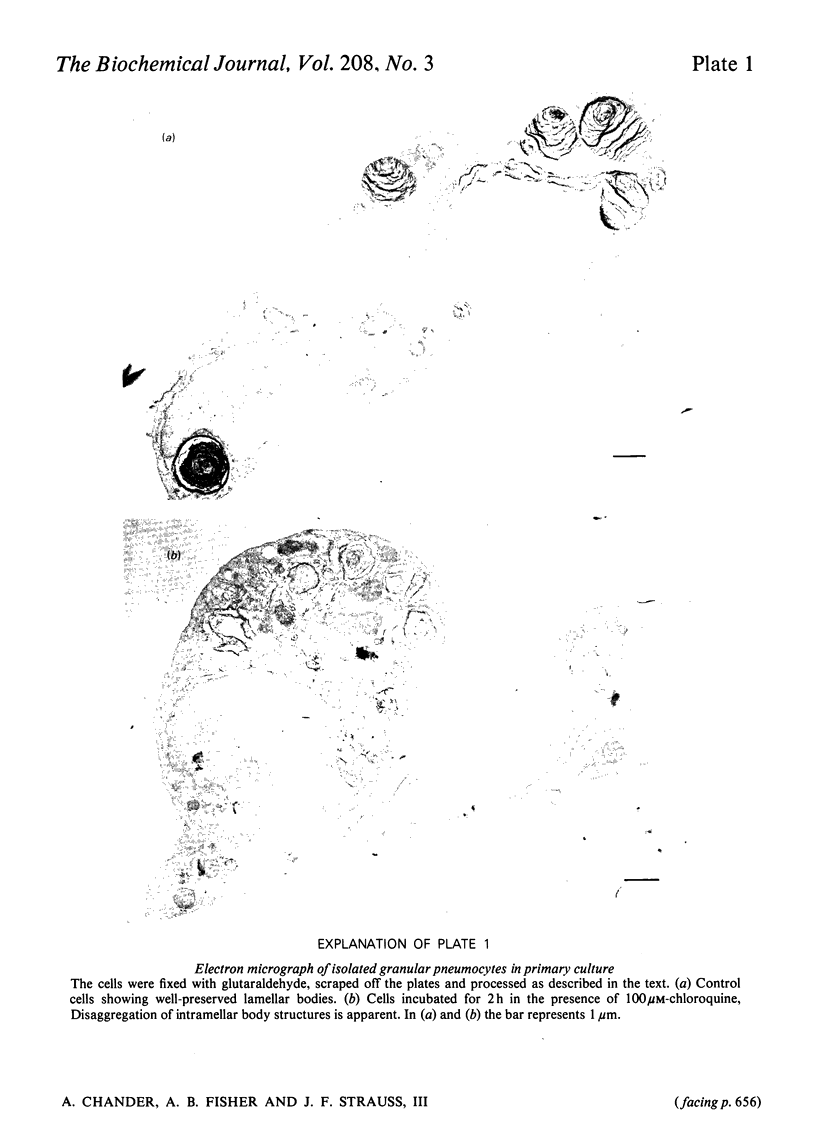
A possible role for an acidic subcellular compartment in biosynthesis of lung surfactant phospholipids was evaluated with granular pneumocytes in primary culture. Incubation with chloroquine (100μm) was used to perturb this compartment. With control cells, incorporation of [9,10-3H]palmitic acid into total lipids and into total phosphatidylcholines increased linearly with time up to 4h. Total incorporation into phosphatidylcholine during a 1h incubation was 999+85pmol of [9,10-3H]palmitic acid, 458±18pmol of [1-14C]oleic acid and 252±15pmol of [U-14C]glucose per μg of phosphatidylcholine phosphorus. The cellular content of either disaturated phosphatidylcholine or total phosphatidylcholines did not change during a 2h incubation with chloroquine. In the presence of chloroquine, the specific radioactivity of [3H]palmitic acid in disaturated phosphatidylcholine increased by 40%, and that of disaturated-phosphatidylcholine fatty acids from [U-14C]glucose increased by 125%. Incorporation of [1-14C]oleic acid into phosphatidylcholine was decreased by chloroquine by 79% and 33% in the presence or absence of palmitic acid respectively. Chloroquine stimulated phospholipase activity in intact cells, and in sonicated cells at pH4.0, but not at pH8.5. The observations indicate that chloroquine stimulates synthesis of disaturated phosphatidylcholine in granular pneumocytes from fatty acids, both exogenous and synthesized de novo, which can be due to stimulation of acidic phospholipase. This stimulation of acidic phospholipase A activity by chloroquine appears to be coupled to the synthesis of disaturated phosphatidylcholine, thereby enhancing remodelling of phosphatidylcholine synthesized de novo. Our findings, therefore, implicate the involvement of an acidic subcellular compartment in the remodelling pathway of disaturated phosphatidylcholine synthesis by granular pneumocytes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMSON D., BLECHER M. QUANTITATIVE TWO-DIMENSIONAL THIN-LAYER CHROMATOGRAPHY OF NATURALLY OCCURRING PHOSPHOLIPIDS. J Lipid Res. 1964 Oct;5:628–631. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Barańska J., van Golde L. M. Role of lamellar bodies in the biosynthesis of phosphatidylcholine in mouse lung. Biochim Biophys Acta. 1977 Aug 24;488(2):285–293. doi: 10.1016/0005-2760(77)90186-2. [DOI] [PubMed] [Google Scholar]
- Batenburg J. J., Longmore W. J., van Golde L. M. The synthesis of phosphatidylcholine by adult rat lung alveolar type II epithelial cells in primary culture. Biochim Biophys Acta. 1978 Apr 28;529(1):160–170. doi: 10.1016/0005-2760(78)90114-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown L. A., Longmore W. J. Adrenergic and cholinergic regulation of lung surfactant secretion in the isolated perfused rat lung and in the alveolar type II cell in culture. J Biol Chem. 1981 Jan 10;256(1):66–72. [PubMed] [Google Scholar]
- DiAugustine R. P. Lung concentric laminar organelle. Hydrolase activity and compositional analysis. J Biol Chem. 1974 Jan 25;249(2):584–593. [PubMed] [Google Scholar]
- Dicorleto P. E., Fakharzadeh F. F., Searles L. L., Zilversmit D. B. Stimulation by acidic phospholipids of protein-catalyzed phosphatidylcholine transfer. Biochim Biophys Acta. 1977 Jul 14;468(2):296–304. doi: 10.1016/0005-2736(77)90122-5. [DOI] [PubMed] [Google Scholar]
- Engle M. J., Sanders R. L., Longmore W. J. Evidence for the synthesis of lung surfactant dipalmitoyl phosphatidylcholine by a "remodeling" mechanism. Biochem Biophys Res Commun. 1980 May 14;94(1):23–28. doi: 10.1016/s0006-291x(80)80181-1. [DOI] [PubMed] [Google Scholar]
- Engle M. J., Sanders R. L., Longmore W. J. Phospholipid composition and acyltransferase activity of lamellar bodies isolated from rat lung. Arch Biochem Biophys. 1976 Apr;173(2):586–595. doi: 10.1016/0003-9861(76)90295-2. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Finkelstein J. N., Mavis R. D. Biochemical evidence for internal proteolytic damage during isolation of type II alveolar epithelial cells. Lung. 1979;156(4):243–254. doi: 10.1007/BF02714018. [DOI] [PubMed] [Google Scholar]
- Fisher A. B., Furia L., Berman H. Metabolism of rat granular pneumocytes isolated in primary culture. J Appl Physiol Respir Environ Exerc Physiol. 1980 Oct;49(4):743–750. doi: 10.1152/jappl.1980.49.4.743. [DOI] [PubMed] [Google Scholar]
- Gross I., Warshaw J. B. Enzyme activities related to fatty acid synthesis in developing mammalian lung. Pediatr Res. 1974 Mar;8(3):193–199. doi: 10.1203/00006450-197403000-00007. [DOI] [PubMed] [Google Scholar]
- Harder A., Kovatchev S., Debuch H. Interactions of chloroquine with different glycerophospholipids. Hoppe Seylers Z Physiol Chem. 1980 Dec;361(12):1847–1850. [PubMed] [Google Scholar]
- Heath M. F., Jacobson W. Phospholipases A1 and A2 in lamellar inclusion bodies of the alveolar epithelium of rabbit lung. Biochim Biophys Acta. 1976 Sep 27;441(3):443–452. doi: 10.1016/0005-2760(76)90241-1. [DOI] [PubMed] [Google Scholar]
- Heath M. F., Jacobson W. The action of lung lysosomal phospholipases on dipalmitoyl phosphatidylcholine and its significance for the synthesis of pulmonary surfactant. Pediatr Res. 1980 Mar;14(3):254–258. doi: 10.1203/00006450-198003000-00016. [DOI] [PubMed] [Google Scholar]
- Heath M. F., Jacobson W. The nature of the phospholipases A of lung lamellar bodies. Pediatr Res. 1980 Jun;14(6):846–847. doi: 10.1203/00006450-198006000-00016. [DOI] [PubMed] [Google Scholar]
- Kikkawa Y., Yoneda K. The type II epithelial cell of the lung. I. Method of isolation. Lab Invest. 1974 Jan;30(1):76–84. [PubMed] [Google Scholar]
- King R. J. Utilization of alveolar epithelial type II cells for the study of pulmonary surfactant. Fed Proc. 1979 Nov;38(12):2637–2643. [PubMed] [Google Scholar]
- Longmore W. J., Oldenborg V., van Golde L. M. Phospholipase A2 in rat-lung microsomes: substrate specificity towards endogenous phosphatidylcholines. Biochim Biophys Acta. 1979 Mar 29;572(3):452–460. doi: 10.1016/0005-2760(79)90152-8. [DOI] [PubMed] [Google Scholar]
- Markus H. B., Ball E. G. Inhibition of lipolytic processes in rat adipose tissue by antimalaria drugs. Biochim Biophys Acta. 1969 Dec 17;187(4):486–491. doi: 10.1016/0005-2760(69)90045-9. [DOI] [PubMed] [Google Scholar]
- Mason R. J., Dobbs L. G. Synthesis of phosphatidylcholine and phosphatidylglycerol by alveolar type II cells in primary culture. J Biol Chem. 1980 Jun 10;255(11):5101–5107. [PubMed] [Google Scholar]
- Mason R. J., Nellenbogen J., Clements J. A. Isolation of disaturated phosphatidylcholine with osmium tetroxide. J Lipid Res. 1976 May;17(3):281–284. [PubMed] [Google Scholar]
- Matsuzawa Y., Hostetler K. Y. Inhibition of lysosomal phospholipase A and phospholipase C by chloroquine and 4,4'-bis(diethylaminoethoxy) alpha, beta-diethyldiphenylethane. J Biol Chem. 1980 Jun 10;255(11):5190–5194. [PubMed] [Google Scholar]
- Moriya T., Kano H. In vivo studies on the de novo synthesis of molecular species of rat lung lecithins. Tohoku J Exp Med. 1974 Mar;112(3):241–256. doi: 10.1620/tjem.112.241. [DOI] [PubMed] [Google Scholar]
- Seglen P. O., Grinde B., Solheim A. E. Inhibition of the lysosomal pathway of protein degradation in isolated rat hepatocytes by ammonia, methylamine, chloroquine and leupeptin. Eur J Biochem. 1979 Apr 2;95(2):215–225. doi: 10.1111/j.1432-1033.1979.tb12956.x. [DOI] [PubMed] [Google Scholar]
- Smith F. B., Kikkawa Y. The type II epithelial cells of the lung. V. Synthesis of phosphatidyl glycerol in isolated type II cells and pulmonary alveolar macrophages. Lab Invest. 1979 Feb;40(2):172–177. [PubMed] [Google Scholar]
- Tjiong H. B., Lepthin J., Debuch H. Lysosomal phospholipids from rat liver after treatment with different drugs. Hoppe Seylers Z Physiol Chem. 1978 Jan;359(1):63–69. doi: 10.1515/bchm.1978.359.1.63. [DOI] [PubMed] [Google Scholar]
- Tsao F. H., Zachman R. D. Phosphatidylcholine-lysophosphatidylcholine cycle pathway enzymes in rabbit lung. I. Subcellular localization and properties. Pediatr Res. 1977 Jul;11(7):849–857. doi: 10.1203/00006450-197707000-00015. [DOI] [PubMed] [Google Scholar]
- Van Golde L. M. Metabolism of phospholipids in the lung. Am Rev Respir Dis. 1976 Nov;114(5):977–1000. doi: 10.1164/arrd.1976.114.5.977. [DOI] [PubMed] [Google Scholar]
- de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974 Sep 15;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]