Abstract
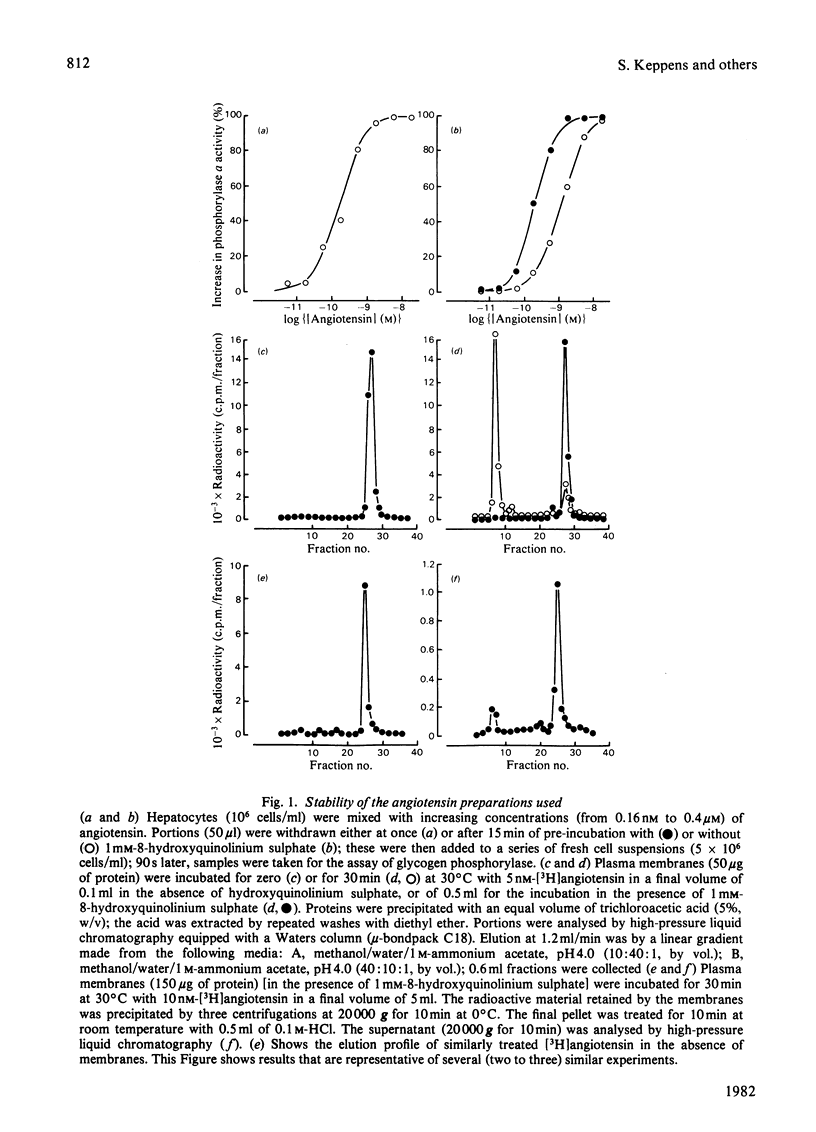
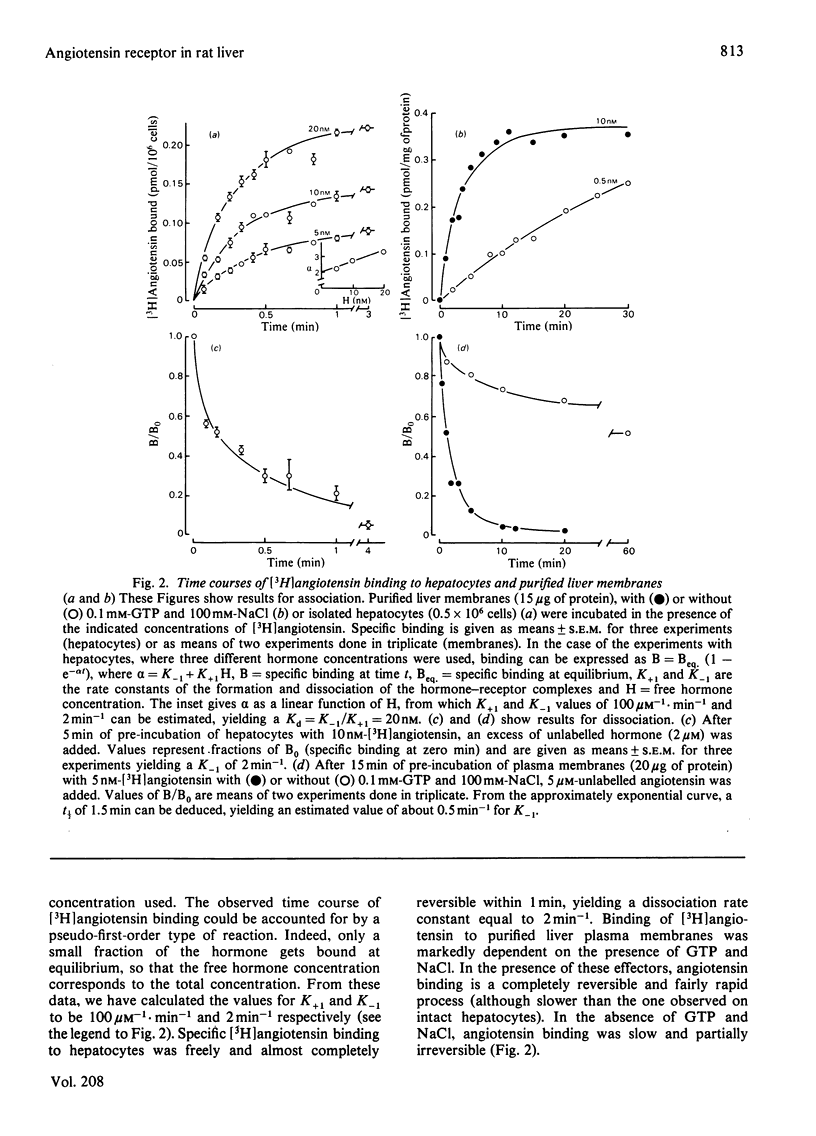
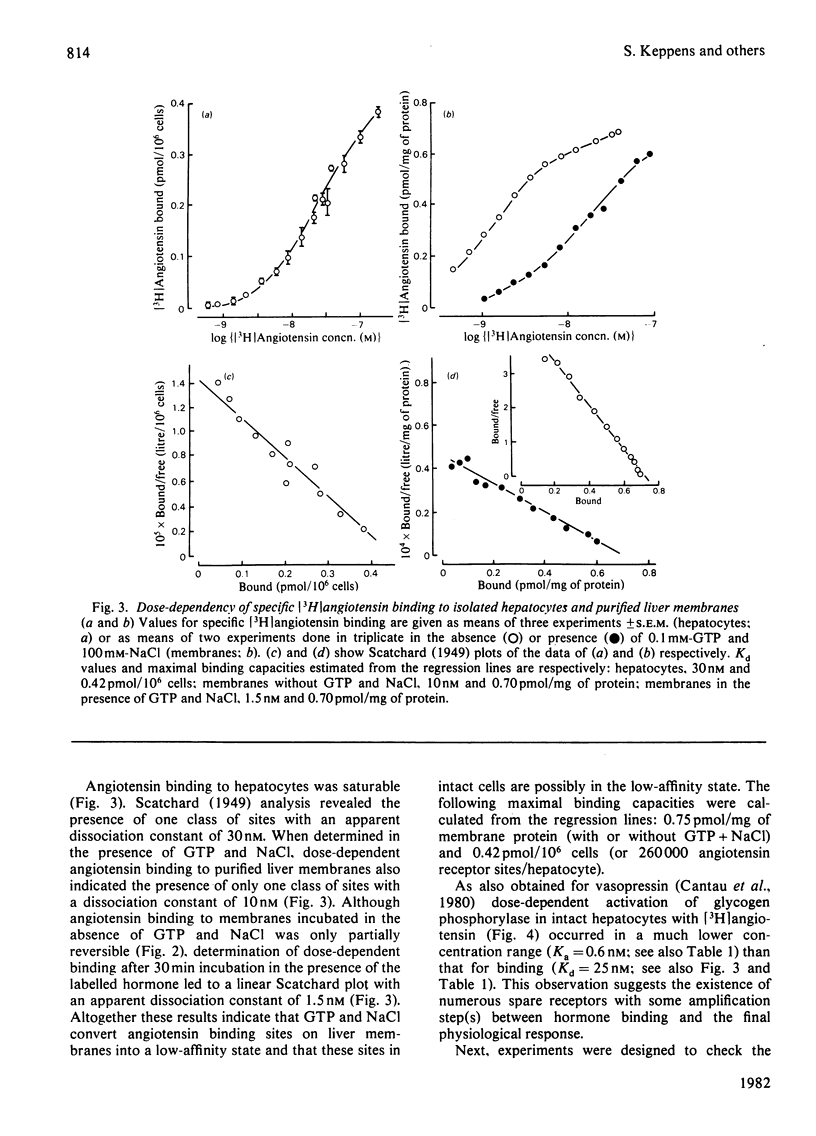
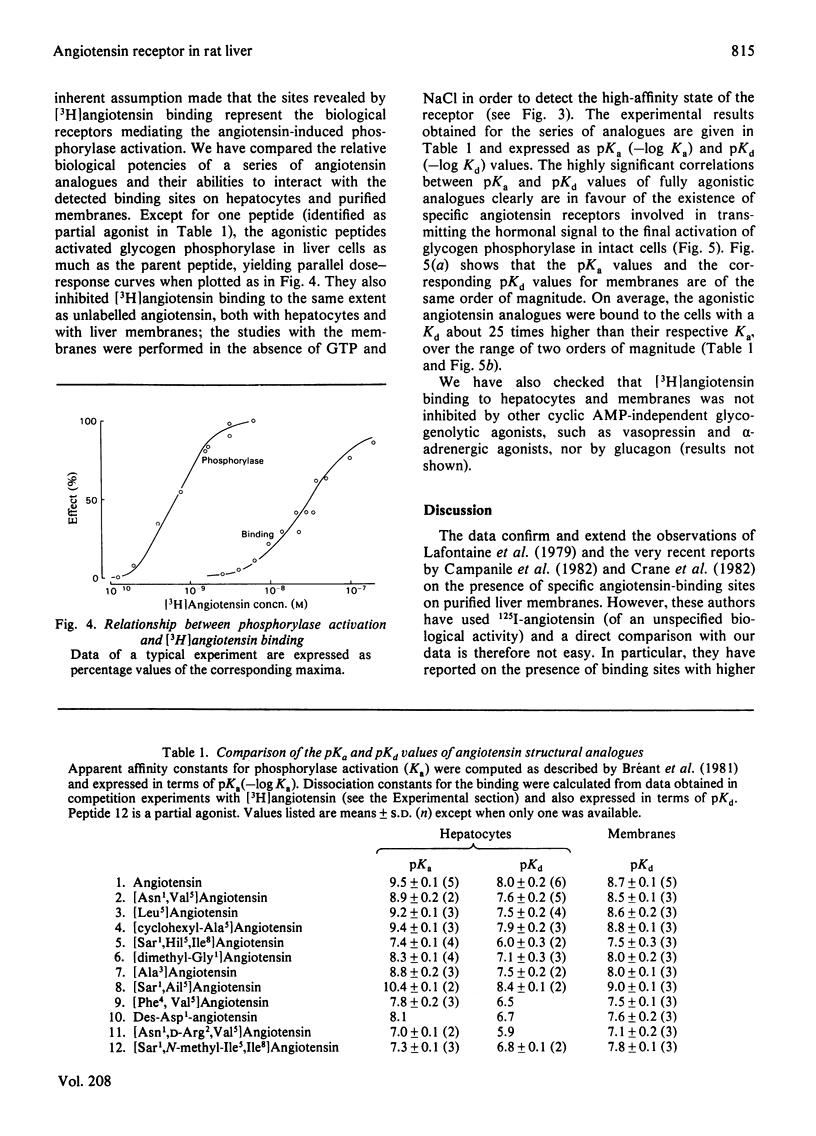
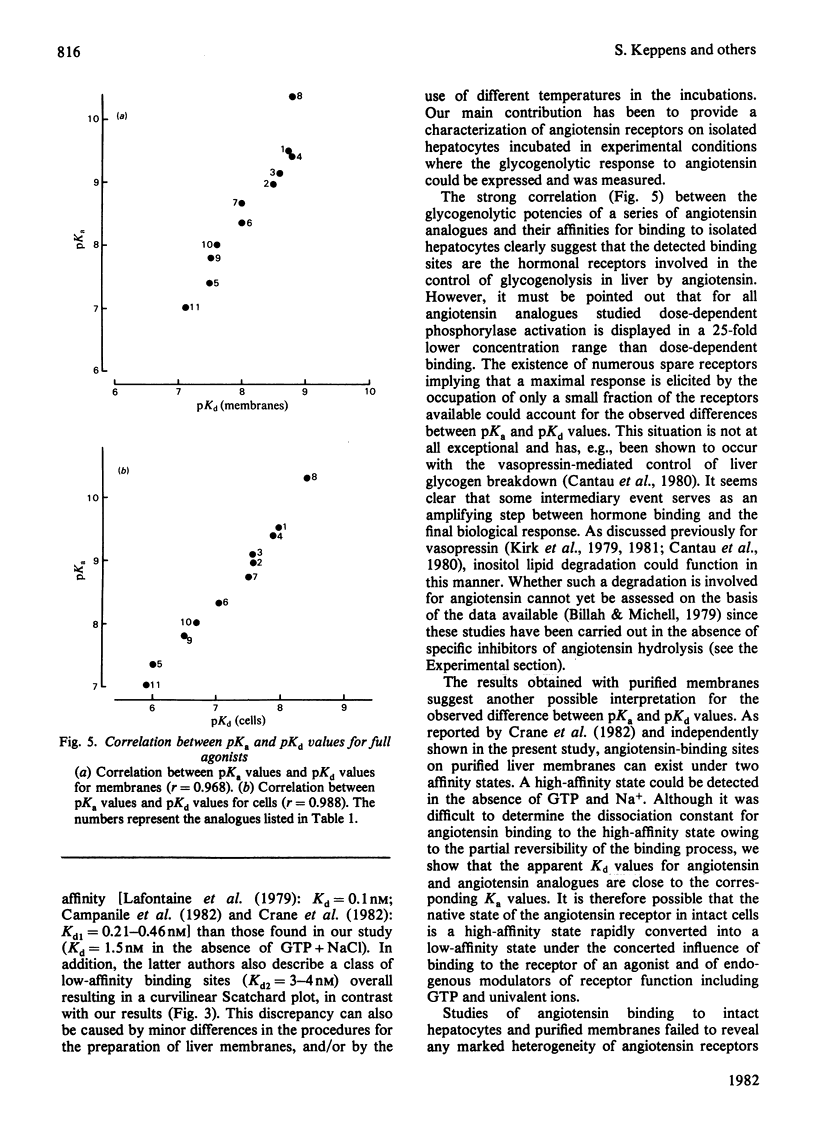
Specific angiotensin binding to rat hepatocytes and purified liver plasma membranes was measured by using biologically active [3H]angiotensin (sp. radioactivity 14Ci/mmol). The kinetic parameters for angiotensin binding to hepatocytes are: K+1 (association rate constant). 100μm−1·min−1; K−1 (dissociation rate constant), 2min−1; Kd (dissociation constant). 30nm; maximal binding capacity, 0.42pmol/106 cells or 260000 sites/cell. Angiotensin binding to membranes is profoundly affected by GTP (0.1mm) and NaCl (100mm); these regulatory compounds greatly enhance both the rate of association and of dissociation and also the extent of dissociation. Kd amounts to 10nm in the presence of GTP+NaCl and to 1.5nm in their absence; maximal binding capacity is 0.70pmol/mg of protein, both with or without GTP+NaCl. The relative affinities of 11 angiotensin structural analogues were deduced from competition experiments for [3H]angiotensin binding to hepatocytes and to membranes (in the latter case, GTP + NaCl were not included, in order to study the higher affinity state of the receptor). These are highly correlated with their biological activity (activation of glycogen phosphorylase in hepatocytes). Binding to membranes occurs in the same concentration range as the biological effect. On the other hand, the existence of numerous spare receptors is suggested by the observation that binding of the agonists to hepatocytes requires 25-fold higher concentrations than those needed for their biological activity. These data clearly suggest that the detected binding sites correspond to the physiological receptors involved in the glycogenolytic action of angiotensin on rat liver.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billah M. M., Michell R. H. Phosphatidylinositol metabolism in rat hepatocytes stimulated by glycogenolytic hormones. Effects of angiotensin, vasopressin, adrenaline, ionophore A23187 and calcium-ion deprivation. Biochem J. 1979 Sep 15;182(3):661–668. doi: 10.1042/bj1820661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bréant B., Keppens S., De Wulf H. Heterologous desensitization of the cyclic AMP-independent glycogenolytic response in rat liver cells. Biochem J. 1981 Dec 15;200(3):509–514. doi: 10.1042/bj2000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanile C. P., Crane J. K., Peach M. J., Garrison J. C. The hepatic angiotensin II receptor. I. Characterization of the membrane-binding site and correlation with physiological response in hepatocytes. J Biol Chem. 1982 May 10;257(9):4951–4958. [PubMed] [Google Scholar]
- Cantau B., Keppens S., De Wulf H., Jard S. (3H)-vasopressin binding to isolated rat hepatocytes and liver membranes: regulation by GTP and relation to glycogen phosphorylase activation. J Recept Res. 1980;1(2):137–168. doi: 10.3109/10799898009044096. [DOI] [PubMed] [Google Scholar]
- Crane J. K., Campanile C. P., Garrison J. C. The hepatic angiotensin II receptor. II. Effect of guanine nucleotides and interaction with cyclic AMP production. J Biol Chem. 1982 May 10;257(9):4959–4965. [PubMed] [Google Scholar]
- Exton J. H. Molecular mechanisms involved in alpha-adrenergic responses. Mol Cell Endocrinol. 1981 Sep;23(3):233–264. doi: 10.1016/0303-7207(81)90123-4. [DOI] [PubMed] [Google Scholar]
- Fain J. N., García-Sáinz J. A. Role of phosphatidylinositol turnover in alpha 1 and of adenylate cyclase inhibition in alpha 2 effects of catecholamines. Life Sci. 1980 Apr 14;26(15):1183–1194. doi: 10.1016/0024-3205(80)90062-4. [DOI] [PubMed] [Google Scholar]
- Jard S., Cantau B., Jakobs K. H. Angiotensin II and alpha-adrenergic agonists inhibit rat liver adenylate cyclase. J Biol Chem. 1981 Mar 25;256(6):2603–2606. [PubMed] [Google Scholar]
- Keppens S., de Wulf H. The nature of the hepatic receptors involved in vasopressin-induced glycogenolysis. Biochim Biophys Acta. 1979 Nov 15;588(1):63–69. doi: 10.1016/0304-4165(79)90371-4. [DOI] [PubMed] [Google Scholar]
- Kirk C. J., Creba J. A., Downes C. P., Michell R. H. Hormone-stimulated metabolism of inositol lipids and its relationship to hepatic receptor function. Biochem Soc Trans. 1981 Oct;9(5):377–379. doi: 10.1042/bst0090377. [DOI] [PubMed] [Google Scholar]
- Kirk C. J., Rodrigues L. M., Hems D. A. The influence of vasopressin and related peptides on glycogen phosphorylase activity and phosphatidylinositol metabolism in hepatocytes. Biochem J. 1979 Feb 15;178(2):493–496. doi: 10.1042/bj1780493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lafontaine J. J., Nivez M. P., Ardaillou R. Hepatic binding sites for angiotensen II in the rat. Clin Sci (Lond) 1979 Jan;56(1):33–40. doi: 10.1042/cs0560033. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Billah M. M. Hormonal stimulation of phosphatidylinositol breakdown with particular reference to the hepatic effects of vasopressin. Biochem Soc Trans. 1979 Oct;7(5):861–865. doi: 10.1042/bst0070861. [DOI] [PubMed] [Google Scholar]
- Morgat J. L., Hung L. T., Fromageot P. Preparation of highly labelled (3H)angiotensin II. Biochim Biophys Acta. 1970 May 26;207(2):374–376. doi: 10.1016/0005-2795(70)90032-2. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Isolation of an organ specific protein antigen from cell-surface membrane of rat liver. Biochim Biophys Acta. 1968 Apr 9;154(3):540–552. doi: 10.1016/0005-2795(68)90014-7. [DOI] [PubMed] [Google Scholar]
- Schmelck P. H., Hanoune J. The hepatic adrenergic receptors. Mol Cell Biochem. 1980 Dec 10;33(1-2):35–48. doi: 10.1007/BF00224570. [DOI] [PubMed] [Google Scholar]
- Vandenheede J. R., Keppens S., De Wulf H. The activation of liver phosphorylase b kinase by glucagon. FEBS Lett. 1976 Jan 15;61(2):213–217. doi: 10.1016/0014-5793(76)81040-x. [DOI] [PubMed] [Google Scholar]


