ABSTRACT
The use of the glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptor agonist tirzepatide has markedly increased for the treatment of weight loss and management of diabetes mellitus. Gastrointestinal adverse effects of GLP-1/GIP agonist therapy, including nausea, vomiting, and constipation, are common. We report a case of colonic ischemia in a 62-year-old woman which developed in association with the use of tirzepatide for weight loss. This report highlights a potential risk relationship between GLP-1/GIP agonist therapy and colonic ischemia and identifies risk factors that should be considered before prescribing tirzepatide.
KEYWORDS: tirzepatide, colonic ischemia, glucagon-like peptide-1 receptor agonists
INTRODUCTION
Colonic ischemia (CI) is the most common form of gastrointestinal ischemic disease and most commonly presents with abrupt onset of abdominal pain followed by bloody diarrhea. The pathophysiology of CI is driven mainly by colonic hypoperfusion in microvessels followed by reperfusion injury.1 There are multiple potential causes of CI including vascular, cardiac, infectious, iatrogenic, and pharmacologic.1 Numerous medications have been associated with CI, including constipation-inducing drugs (eg, opioids, muscarinic antagonists), diuretics (eg, furosemide), nonsteroidal anti-inflammatory drugs, illicit drugs (eg, cocaine), digoxin, hormonal therapies (eg, combined oral contraceptives), and laxatives.2,3 Mechanistic explanations for the development of CI secondary to constipation include reduced efferent vagal cholinergic activity resulting in reduced colonic blood flow, as well as increased intracolonic luminal pressure resulting in reduced colonic blood flow with mucosal-to-serosal shunting of blood.2
Glucagon-like peptide-1 (GLP-1) receptor agonists (RAs) are incretin mimetics used for weight loss and treatment of type 2 diabetes mellitus.4,5 They are generally well tolerated though commonly result in gastrointestinal symptoms, including constipation that can potentially be severe.6–9 Tirzepatide is a novel combined GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) RA approved for treatment of type 2 diabetes mellitus and for weight management. Compared with other GLP-1 RAs, tirzepatide is associated with a similar rate of gastrointestinal adverse events but a higher risk of drug discontinuation due to adverse drug events when used at higher doses (10 or 15 mg subcutaneous once weekly).10,11 In this report, we describe a case of severe constipation followed by CI after the initiation of tirzepatide for weight loss.
CASE REPORT
A 62-year-old woman presented with acute onset abdominal pain and bloody stools. She had a history of obesity (body mass index 40 kg/m2), prior venous thromboembolism, and heart failure with preserved ejection fraction. She was up to date on colorectal cancer screening; previous colonoscopies had shown benign polyps and diverticulosis. Her medications included tirzepatide 2.5 mg subcutaneous once weekly, apixaban 5 mg twice daily, spironolactone 25 mg once daily, furosemide 20 mg once daily, atorvastatin 20 mg once daily, fluoxetine 30 mg once daily, lisinopril 2.5 mg once daily, and atenolol 37.5 mg once daily. She did not take any over-the-counter supplements or laxatives. She had started low-dose tirzepatide (2.5 mg subcutaneous once weekly) for weight loss 4 weeks before presentation and had noted new-onset constipation since then, with firm bowel movements once every 3 days. She had been eating smaller, more frequent meals after starting tirzepatide, but there were otherwise no changes to her diet.
The patient presented with lower abdominal cramping pain, urge to defecate, and bloody stools of 1-day duration. On presentation, she was hypertensive with a blood pressure of 155/103 mm Hg; vital signs were otherwise normal. On physical examination, she had a soft, nondistended abdomen with mild diffuse lower tenderness without guarding. Laboratory studies revealed a white blood cell count of 12.0 (reference range 4.5–11.0 × 109/L) with normal hemoglobin and platelet count. International normalized ratio was 1.6. Computed tomography (CT) scan of the abdomen and pelvis with contrast showed new wall thickening and pericolonic fat stranding involving the splenic flexure and descending colon (Figure 1). The mesenteric vasculature was patent. Colonoscopy demonstrated inflammatory mucosal changes from the mid-sigmoid colon through the distal transverse colon (Figure 2). Biopsies of the abnormal mucosa showed epithelial denudation and sloughing with necrosis associated with crypt withering (damage), hyalinization of the lamina propria, and lamina propria congestion and hemorrhage, all consistent with CI (Figure 3).12
Figure 1.
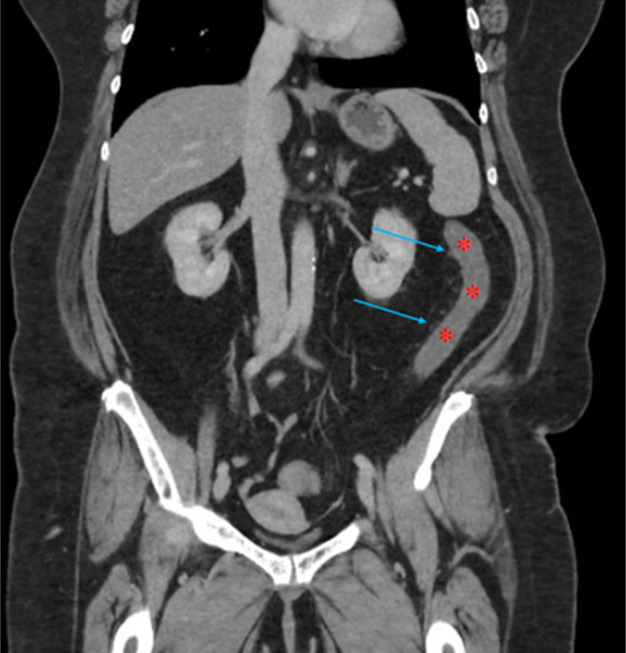
Coronal view of computed tomography scan of the abdomen and pelvis with contrast demonstrating bowel wall thickening (red asterisks) and pericolonic fat stranding (blue arrows) of the distal transverse and descending colon.
Figure 2.
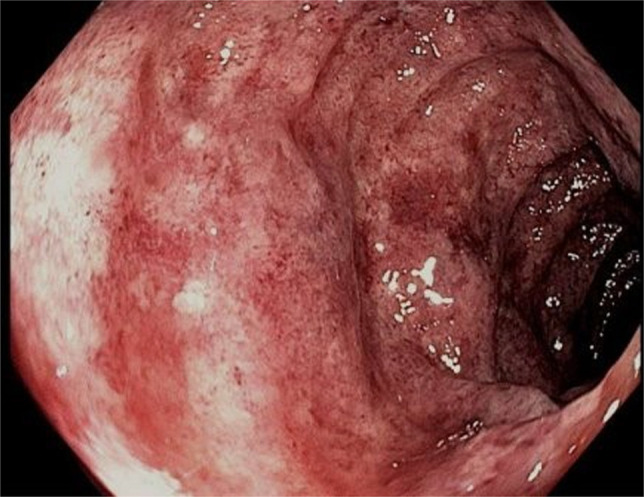
Colonoscopic image showing edematous colonic mucosa with diffuse erythema and areas of pallor.
Figure 3.
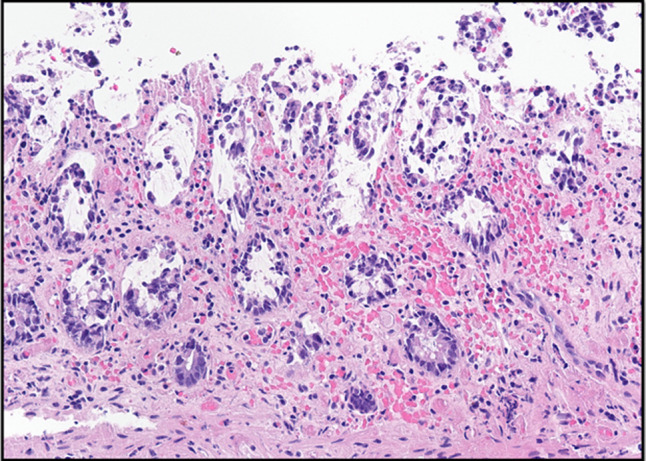
Histopathologic image of colon biopsy stained with hematoxylin and eosin (40×). There is surface epithelium denudation and sloughing with necrosis associated with crypt withering (damage), as well as hyalinization of the lamina propria. The lamina propria also shows congestion and hemorrhage.
The patient was admitted to the hospital and treated with intravenous fluids, bowel rest, broad-spectrum antibiotics (intravenous ciprofloxacin and metronidazole), and analgesics. Apixaban, furosemide, spironolactone, and tirzepatide were held. There was rapid and spontaneous resolution of symptoms, and she was discharged on day 3 of hospitalization. She had been weaned off opioid pain medications more than 24 hours before discharge. Apixaban was resumed at discharge, but diuretics were held. She was discharged with oral ciprofloxacin and metronidazole to complete a 5-day antibiotic course. She had not had any recurrence of rectal bleeding when seen in clinic a week after discharge; however, following this, she had intermittent recurrence of bloody bowel movements for approximately 2 months after discharge. In discussion with gastroenterology, a repeat colonoscopy was performed 2.5 months after hospitalization and found 2 subcentimeter, benign polyps in the transverse colon but otherwise normal mucosa without gross evidence of ischemia or inflammation.
DISCUSSION
The CI, in this case, is most likely multifactorial due to nonocclusive ischemia secondary to tirzepatide-induced constipation, diuretic use, and heart failure. Several features support an association between tirzepatide use and CI. First, the onset of CI was temporally related to the initiation of tirzepatide. Second, new-onset constipation developed while on tirzepatide, which may have been a trigger for the CI episode. In addition, diuretic use likely contributed to the volume depletion and subsequent hypoperfusion in the setting of increased stool burden and baseline reduced cardiac output. Finally, vaso-occlusive etiologies of CI seem less likely in the absence of mesenteric arterial or venous occlusive disease on CT imaging and patent mesenteric vasculature. In addition, the patient reported compliance with her home eliquis therapy and had never had an arterial thrombus, making a cardioembolic cause of CI less likely.
There are only 2 prior case reports in the literature of CI associated with GLP-1 RA therapy. One report occurred in a 45-year-old man who had started tirzepatide 1 week before presenting with watery diarrhea and right upper quadrant abdominal pain.13 The patient had been taking olmesartan-hydrochlorothiazide before presentation. CT imaging findings were consistent with ischemia of the gastric fundus and/or jejunum, with possible colonic involvement. The other report occurred in a 46-year-old woman initiated on semaglutide for weight loss 2 months before presentation.14 The patient reported reduced thirst and appetite after starting a GLP-1 RA and, otherwise, did not have risk factors of intestinal hypoperfusion. The authors of the case conjecture that reduced oral intake caused by the GLP-1 RA may have led to systemic hypotension with resultant CI.14 This report supports that GLP-1 RAs can predispose to CI through more than one mechanism.
Although it is impossible to distinguish association and causation, there is strong rationale for both tirzepatide and GLP-1 RAs to be etiologic for CI. It is likely that the gastrointestinal adverse effects of GLP-1 RAs can contribute to the development of CI by the induction of constipation and volume depletion, the latter of which can result in systemic hypotension which is a known risk factor of CI.1 The risk of CI can be compounded by medications that contribute to either constipation or volume depletion or that are independently associated with CI.2,3 Regarding diuretics, the population-based study by Yadav et al found an odds ratio of 1.6 (95% confidence interval 1.2–2.1) for the development of CI with diuretic use in the 30 days before CI.3 Comorbid medical conditions such as congestive heart failure (associated with an odds ratio of 4.1 for CI in the study of Yadav et al) can further increase the risk of CI in patients started on GLP-1 RAs.3
At this time, data from the literature are too limited to indicate when risk of CI might be the highest following the initiation of tirzepatide or other GLP-1 RA therapy; this may depend on patient and medication factors such as the patient's overall risk for development of CI, as well as the dose of GLP-1 RA therapy. Further study is needed to elucidate the risk relationship between GLP-1 RAs and CI, mechanisms for development of CI with GLP-1 RAs, and whether tirzepatide is associated with a higher risk of CI despite its similar gastrointestinal adverse effect profile when compared with other GLP-1 RAs.10,11 Before prescribing a GLP-1 RA, clinicians should consider risk factors of CI such as a prior episode of CI, peripheral vascular disease, congestive heart failure, and irritable bowel syndrome.1,3,15,16 Our patient was advised to avoid GLP-1 RAs given a potential increased risk of recurrent CI with their use.
To reduce the risk of CI, patients should be counseled to maintain adequate hydration while on a GLP-1 RA provided there are no medical contraindications to increased fluid intake. The patient's medication list should be reviewed, and dose reduction of other medications associated with CI, or that predispose to either hypovolemia or hypotension, should be considered while a patient is on a GLP-1 RA. Clinicians should also be mindful of the risk for constipation with GLP-1 RAs and counsel patients on preventive measures for constipation, as well as prescribe laxative agents when appropriate. Patients with congestive heart failure should be medically optimized, ideally before the initiation of GLP-1 RA therapy.
DISCLOSURES
Author contributions: D. Bayless: writing original draft, investigation, manuscript review and editing; J. Singh: conceptualization, investigation, manuscript review and editing; B. Park: resources, investigation, manuscript review and editing; S. Sweetser: supervision, investigation, manuscript review and editing. S. Sweetser is the article guarantor
Financial disclosure: None to report.
Informed consent was obtained for this case report.
Contributor Information
David Bayless, Email: dbayless14@gmail.com.
Jasraj Singh, Email: marjara.jasraj@mayo.edu.
Byoung Uk Park, Email: park.byoung@mayo.edu.
REFERENCES
- 1.Brandt LJ, Feuerstadt P, Longstreth GF, Boley SJ. ACG clinical guideline: Epidemiology, risk factors, patterns of presentation, diagnosis, and management of colon ischemia(Ci). Am J Gastroenterol. 2015;110(1):18–44. [DOI] [PubMed] [Google Scholar]
- 2.Vodusek Z, Feuerstadt P, Brandt LJ. Review article: The pharmacological causes of colon ischaemia. Aliment Pharmacol Ther. 2019;49(1):51–63. [DOI] [PubMed] [Google Scholar]
- 3.Yadav S, Dave M, Edakkanambeth Varayil J, et al. A population-based study of incidence, risk factors, clinical spectrum, and outcomes of ischemic colitis. Clin Gastroenterol Hepatol. 2015;13(4):731–8.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wojtara M, Mazumder A, Syeda Y, Mozgała N. Glucagon-like peptide-1 receptor agonists for chronic weight management. Adv Med. 2023;2023:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark L. GLP-1 receptor agonists: A review of glycemic benefits and beyond. JAAPA. 2024;37(4):1–4. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Y, Chen M, Liu L, Chen Z. Difference in gastrointestinal risk associated with use of GLP-1 receptor agonists: A real-world pharmacovigilance study. Diabetes Metab Syndr Obes. 2022;15:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu J, Liu H, Zhou Q, Wang MW, Li Z. A potentially serious adverse effect of GLP-1 receptor agonists. Acta Pharm Sin B. 2023;13(5):2291–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon A, Dixit K, Shachi T, Salonia J, Seltzer E. A rare case of a large bowel obstruction due to tirzepatide. Chest. 2023;164(4):A2334–5. [Google Scholar]
- 9.Karrar HR, Nouh MI, Nouh YI, et al. Tirzepatide-induced gastrointestinal manifestations: A systematic review and meta-analysis. Cureus. 2023;15(9):e46091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng Z, Yang M, Wen H, Zhou S, Xiong C, Wang Y. A systematic review of the safety of tirzepatide-a new dual GLP1 and GIP agonist–is its safety profile acceptable? Front Endocrinol. 2023;14:1121387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caruso I, Di Gioia L, Di Molfetta S, et al. The real-world safety profile of tirzepatide: Pharmacovigilance analysis of the FDA adverse event reporting system (Faers) database. J Endocrinol Invest. 2024;47(11):2671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odze RD, Goldblum JR. Surgical Pathology of the GI Tract, Liver, Biliary Tract, and Pancreas. 4th edn. Philadelphia, PA: Elsevier; 2022. [Google Scholar]
- 13.Peicher M, Montes OB, Shekhar R. S3896 the risks of the new weight loss medications. Am J Gastroenterol. 2023;118(10S):S2486. [Google Scholar]
- 14.Wichelmann TA, Kruchko DH, Silas D. S2088 Glucagon-like peptide-1 receptor agonist-associated colonic ischemia: A case report. Am J Gastroenterol. 2022;117(10S):e1424. [Google Scholar]
- 15.Wongtrakul W, Charoenngam N, Ungprasert P. The association between irritable bowel syndrome and ischemic colitis: A systematic review and meta-analysis. Minerva Gastroenterol. 2022;68(4). [DOI] [PubMed] [Google Scholar]
- 16.Cosme A, Montoro M, Santolaria S, et al. Prognosis and follow-up of 135 patients with ischemic colitis over a five-year period. World J Gastroenterol. 2013;19(44):8042–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


