Abstract
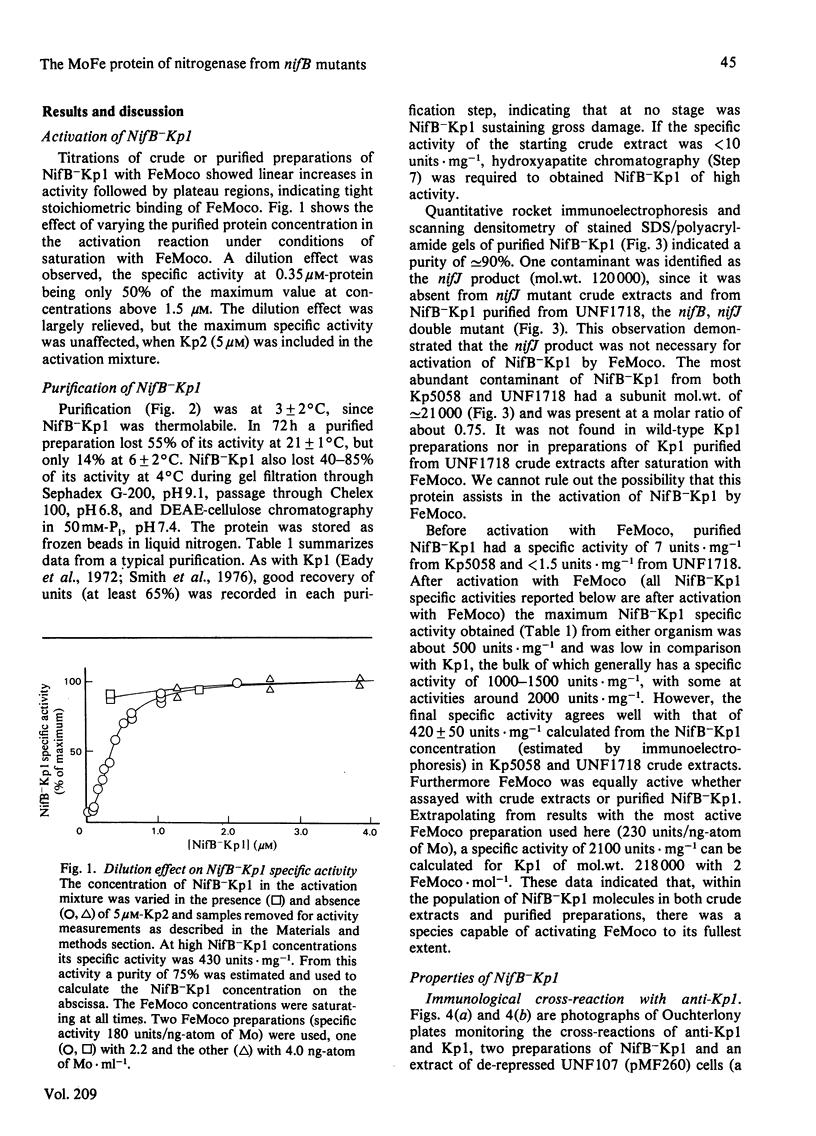
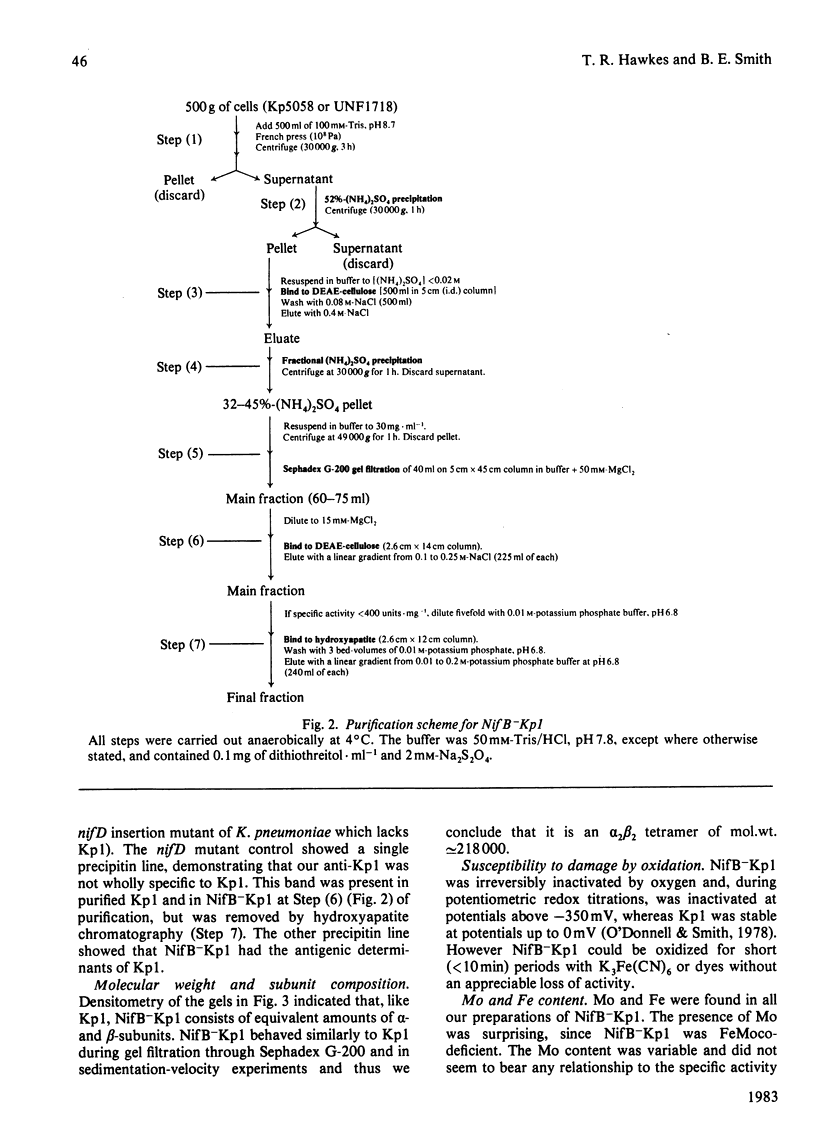
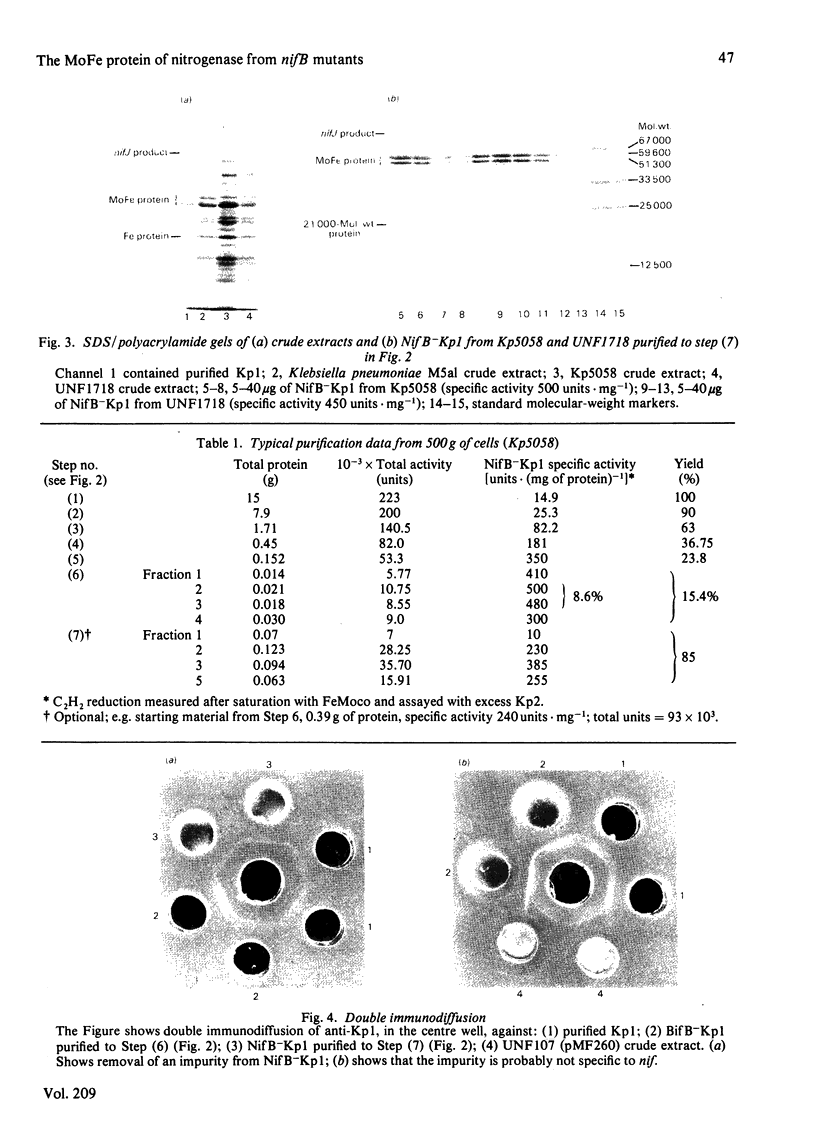
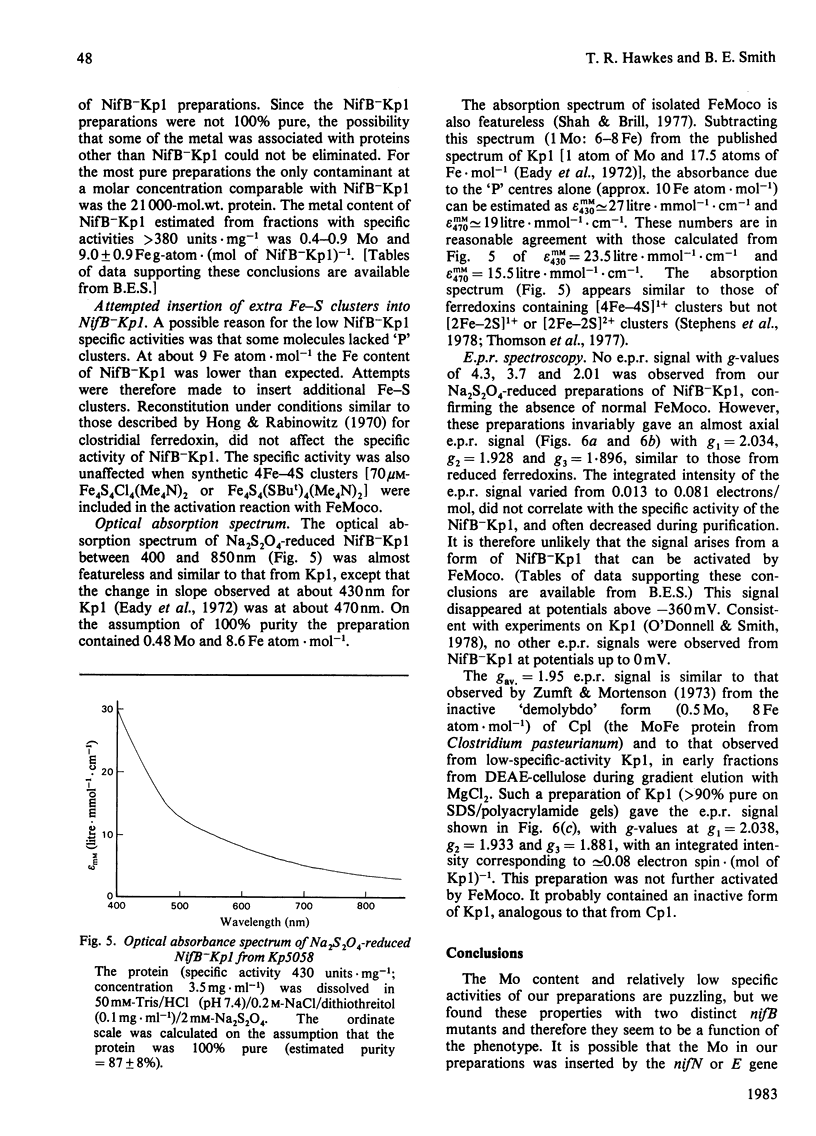
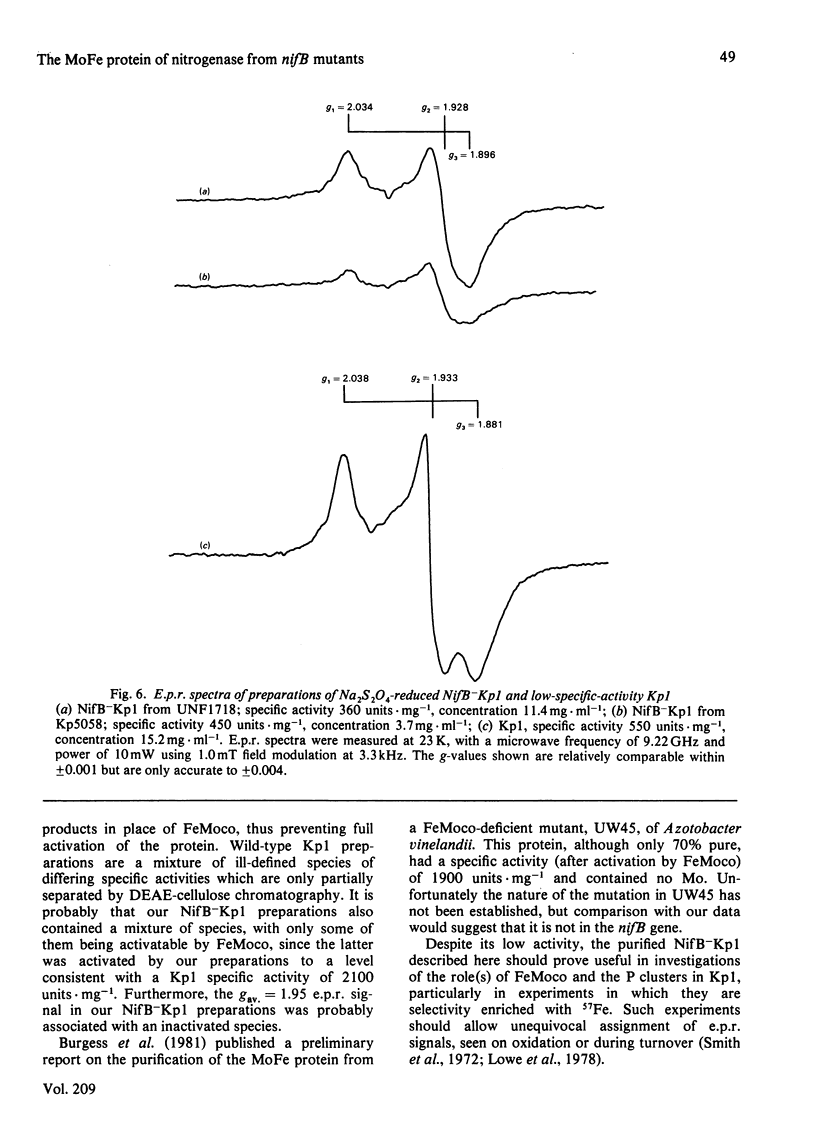
The inactive MoFe protein of nitrogenase, NifB-Kp1, from two distinct nifB mutants of Klebsiella pneumoniae, Kp5058 (a nifB point mutant) and UNF1718 (a nifB, nifJ double mutant) has been purified and characterized. NifB-Kp1 can be activated by reaction with the iron-molybdenum cofactor, FeMoco, extracted from active MoFe protein. NifB-Kp1 purified from either source had similar properties and was contaminated with an approximately equimolar amount of protein of mol.wt. 21 000. Like active wild-type Kp1, it was an alpha 2 beta 2 tetramer, but it was far less stable than Kp1, deteriorating rapidly at temperatures above 8 degrees C or on mild oxidation. NifB-Kp1 preparations contained 0.4-0.9 Mo and 9.0 +/- 0.9 Fe atoms . mol-1 and, when activated by FeMoco, had a specific activity of approx. 500 units . mg-1. The Mo in our preparations was not associated with the e.p.r. signal normally observed from FeMoco. All preparations exhibited a weak gav. = 1.95 e.p.r. signal which was probably not associated with activatable protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess B. K., Jacobs D. B., Stiefel E. I. Large-scale purification of high activity Azotobacter vinelandII nitrogenase. Biochim Biophys Acta. 1980 Jul 10;614(1):196–209. doi: 10.1016/0005-2744(80)90180-1. [DOI] [PubMed] [Google Scholar]
- Dutton P. L. Oxidation-reduction potential dependence of the interaction of cytochromes, bacteriochlorophyll and carotenoids at 77 degrees K in chromatophores of Chromatium D and Rhodopseudomonas gelatinosa. Biochim Biophys Acta. 1971 Jan 12;226(1):63–80. doi: 10.1016/0005-2728(71)90178-2. [DOI] [PubMed] [Google Scholar]
- Eady R. R., Smith B. E., Cook K. A., Postgate J. R. Nitrogenase of Klebsiella pneumoniae. Purification and properties of the component proteins. Biochem J. 1972 Jul;128(3):655–675. doi: 10.1042/bj1280655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. S., Rabinowitz J. C. The all-or-none mode of the reconstitution and the reactions of a,a'-bipyridyl and mercurials with clostridial ferredoxin. J Biol Chem. 1970 Dec 25;245(24):6574–6581. [PubMed] [Google Scholar]
- Huynh B. H., Henzl M. T., Christner J. A., Zimmermann R., Orme-Johnson W. H., Münck E. Nitrogenase XII. Mössbauer studies of the MoFe protein from Clostridium pasteurianum W5. Biochim Biophys Acta. 1980 May 29;623(1):124–138. doi: 10.1016/0005-2795(80)90015-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowe D. J., Eady R. R., Thorneley N. F. Electron-paramagnetic-resonance studies on nitrogenase of Klebsiella pneumoniae. Evidence for acetylene- and ethylene-nitrogenase transient complexes. Biochem J. 1978 Jul 1;173(1):277–290. doi: 10.1042/bj1730277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. J., Eady R. R., Thorneley N. F. Electron-paramagnetic-resonance studies on nitrogenase of Klebsiella pneumoniae. Evidence for acetylene- and ethylene-nitrogenase transient complexes. Biochem J. 1978 Jul 1;173(1):277–290. doi: 10.1042/bj1730277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortenson L. E., Thorneley R. N. Structure and function of nitrogenase. Annu Rev Biochem. 1979;48:387–418. doi: 10.1146/annurev.bi.48.070179.002131. [DOI] [PubMed] [Google Scholar]
- Münck E., Rhodes H., Orme-Johnson W. H., Davis L. C., Brill W. J., Shah V. K. Nitrogenase. VIII. Mössbauer and EPR spectroscopy. The MoFe protein component from Azotobacter vinelandii OP. Biochim Biophys Acta. 1975 Jul 21;400(1):32–53. doi: 10.1016/0005-2795(75)90124-5. [DOI] [PubMed] [Google Scholar]
- O'Donnell M. J., Smith B. E. Electron-paramagnetic-resonance studies on the redox properties of the molybdenum-iron protein of nitrogenase between +50 and -450 mV. Biochem J. 1978 Sep 1;173(3):831–838. doi: 10.1042/bj1730831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings J., Shah V. K., Chisnell J. R., Brill W. J., Zimmermann R., Münck E., Orme-Johnson W. H. Novel metal cluster in the iron-molybdenum cofactor of nitrogenase. Spectroscopic evidence. J Biol Chem. 1978 Feb 25;253(4):1001–1004. [PubMed] [Google Scholar]
- Shah V. K., Brill W. J. Isolation of a molybdenum--iron cluster from nitrogenase. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3438–3440. doi: 10.1073/pnas.78.6.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V. K., Brill W. J. Isolation of an iron-molybdenum cofactor from nitrogenase. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3249–3253. doi: 10.1073/pnas.74.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. E., Lowe D. J., Bray R. C. Nitrogenase of Klebsiella pneumoniae: electron-paramagnetic-resonance studies on the catalytic mechanism. Biochem J. 1972 Nov;130(2):641–643. doi: 10.1042/bj1300641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens P. J., Thomson A. J., Dunn J. B., Keiderling T. A., Rawlings J., Rao K. K., Hall D. O. Circular dichroism and magnetic circular dichroism of iron-sulfur proteins. Biochemistry. 1978 Oct 31;17(22):4770–4778. doi: 10.1021/bi00615a026. [DOI] [PubMed] [Google Scholar]
- Thomson A. J., Cammack R., Hall D. O., Rao K. K., Briat B., Rivoal J. C., Badoz J. The low temperature magnetic circular dichroism spectra of iron-sulphur proteins. II. Two-iron ferredoxins. Biochim Biophys Acta. 1977 Jul 22;493(1):132–141. doi: 10.1016/0005-2795(77)90266-5. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Zumft W. G., Mortensson L. E. Evidence for a catalytic-centre heterogeneity of molybdoferredoxin from Clostridium pasteurianum. Eur J Biochem. 1973 Jun 15;35(3):401–409. doi: 10.1111/j.1432-1033.1973.tb02852.x. [DOI] [PubMed] [Google Scholar]




