Abstract
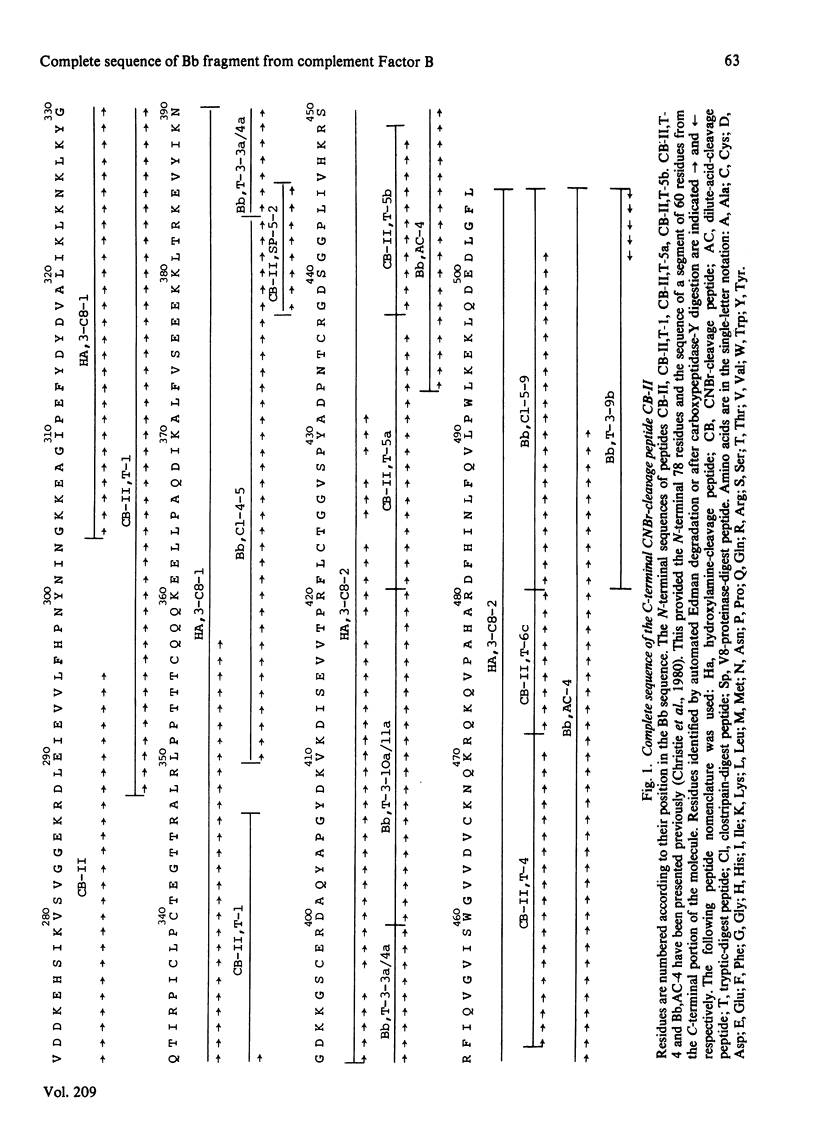
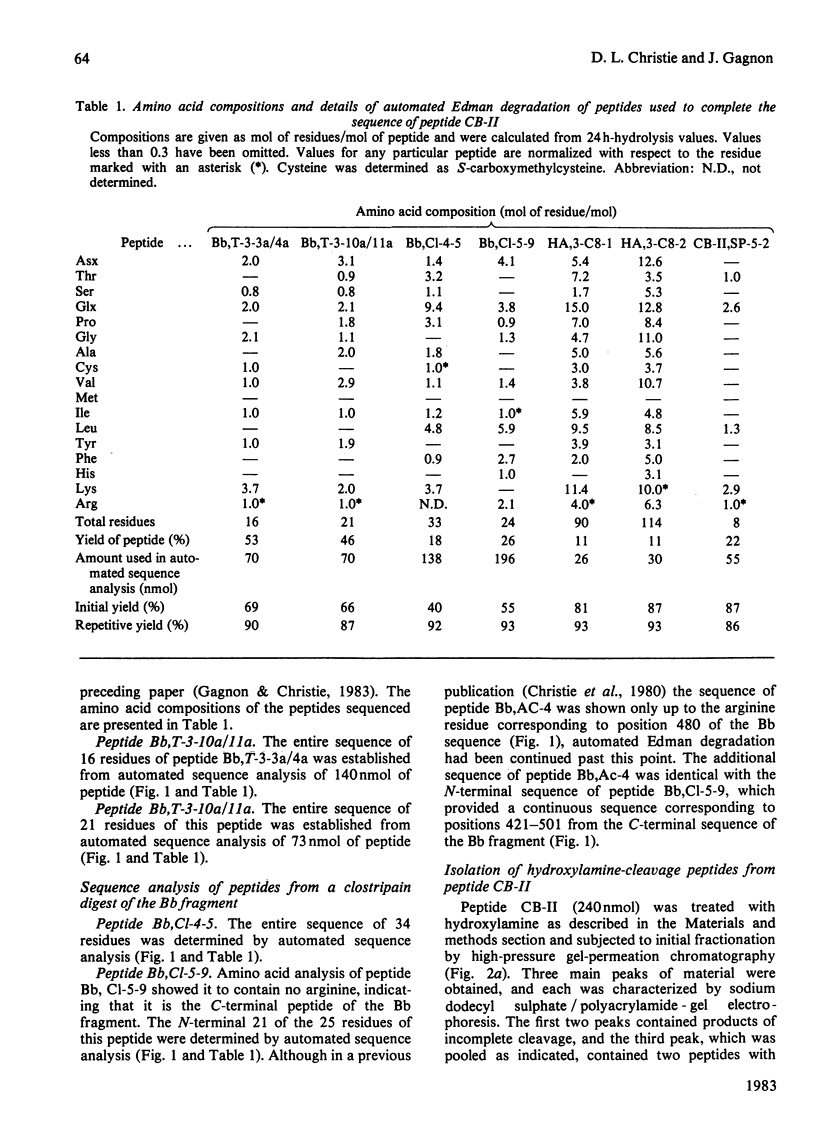
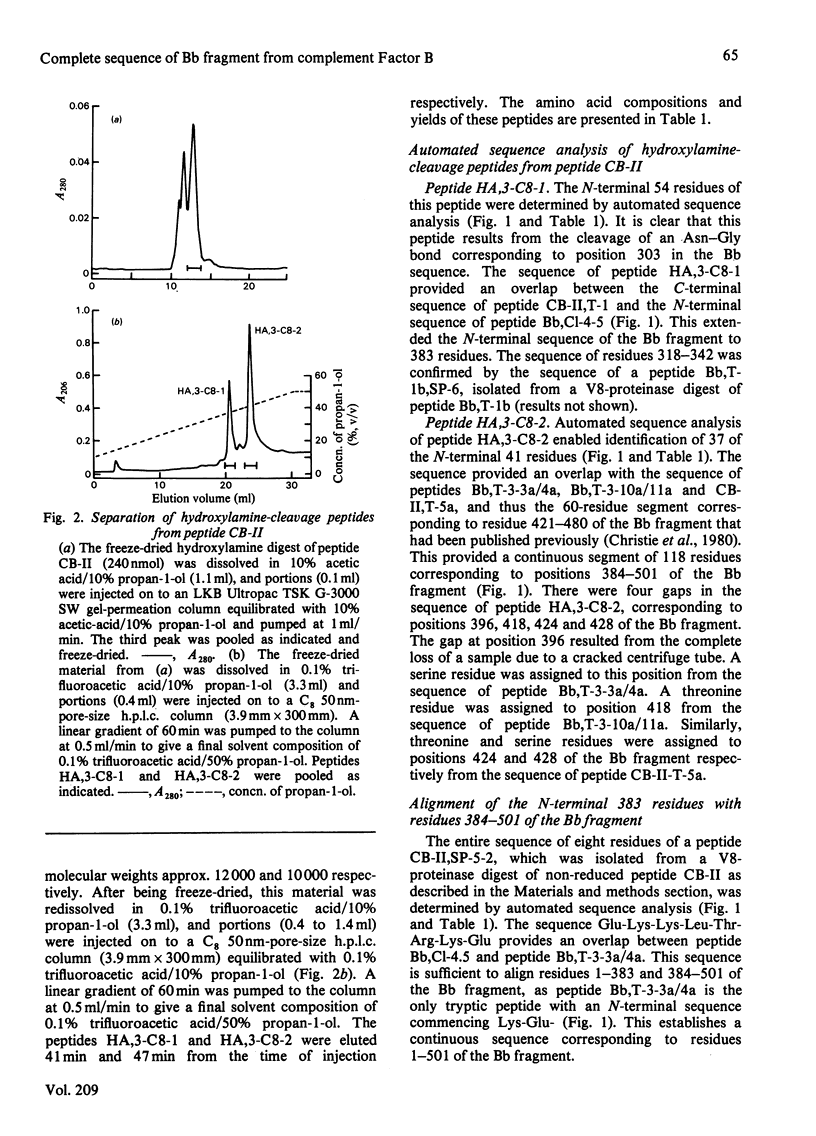
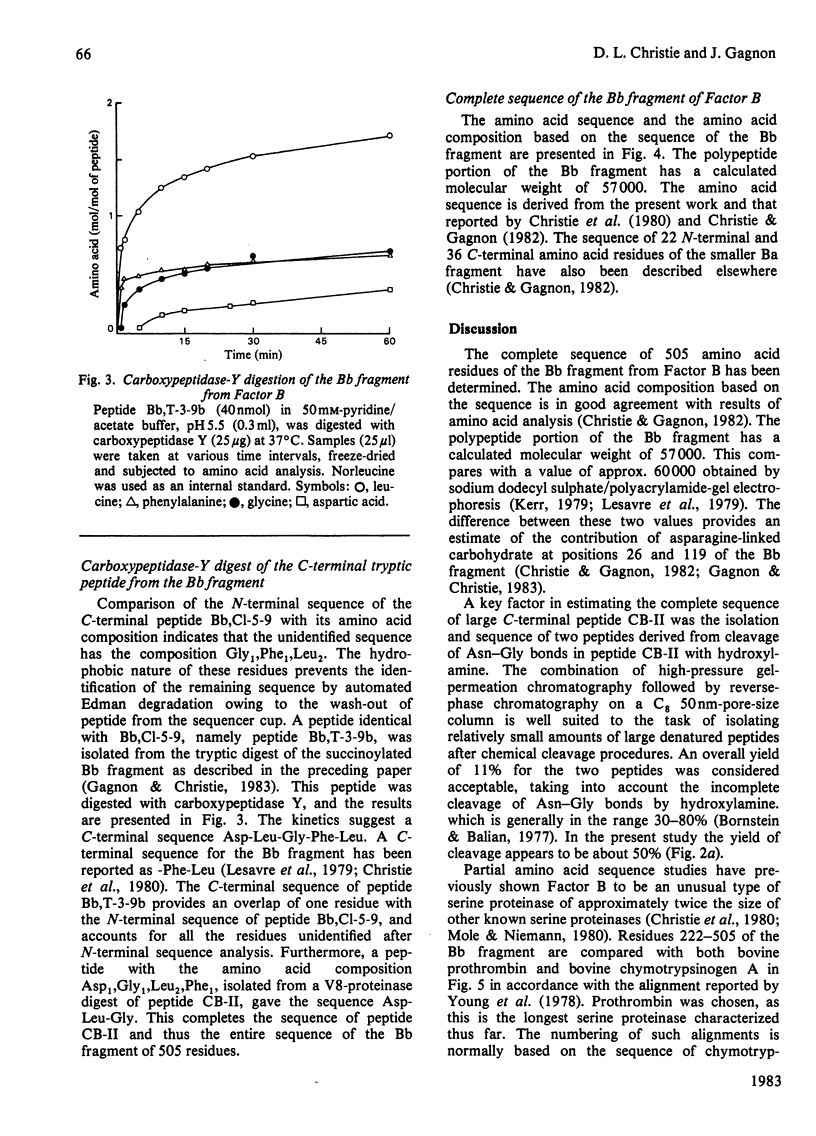
The amino acid sequence of peptide CB-II, the major product (mol.wt. 30 000) of CNBr cleavage of fragment Bb from human complement Factor B, is given. The sequence was obtained from peptides derived by trypsin cleavage of peptide CB-II and clostripain digestion of fragment Bb. Cleavage of two Asn-Gly bonds in peptide CB-II was also found useful. These results, along with those presented in the preceding paper [Gagnon & Christie (1983) Biochem. J. 209, 51-60], yield the complete sequence of the 505 amino acid residues of fragment Bb. The C-terminal half of the molecule shows strong homology of sequence with serine proteinases. Factor B has a catalytic chain (fragment Bb) with a molecular weight twice that of proteinases previously described, suggesting that it is a novel type of serine proteinase, probably with a different activation mechanism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arlaud G. J., Gagnon J. Clr and Cls subcomponents of human complement: two serine proteinases lacking the 'histidine-loop' disulphide bridge. Biosci Rep. 1981 Oct;1(10):779–784. doi: 10.1007/BF01114800. [DOI] [PubMed] [Google Scholar]
- Arlaud G. J., Gagnon J., Porter R. R. The catalytic chain of human complement subcomponent C1r. Purification and N-terminal amino acid sequences of the major cyanogen bromide-cleavage fragments. Biochem J. 1982 Jan 1;201(1):49–59. doi: 10.1042/bj2010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balian G., Click E. M., Bornstein P. Structure of rat skin collagen 1-CB8. Amino acid sequence of the hydroxylamine-produced fragment HA1. Biochemistry. 1971 Nov 23;10(24):4470–4478. doi: 10.1021/bi00800a019. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Balian G. Cleavage at Asn-Gly bonds with hydroxylamine. Methods Enzymol. 1977;47:132–145. doi: 10.1016/0076-6879(77)47016-2. [DOI] [PubMed] [Google Scholar]
- Christie D. L., Gagnon J. Isolation, characterization and N-terminal sequences of the CNBr-cleavage peptides from human complement Factor B. Localization of a free thiol group and a sequence defining the site cleaved by factor D. Biochem J. 1982 Mar 1;201(3):555–567. doi: 10.1042/bj2010555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie D. L., Gagnon J., Porter R. R. Partial sequence of human complement component factor B: novel type of serine protease. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4923–4927. doi: 10.1073/pnas.77.8.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curman B., Sandberg-Trägårdh L., Peterson P. A. Chemical characterization of human factor B of the alternate pathway of complement activation. Biochemistry. 1977 Nov 29;16(24):5368–5375. doi: 10.1021/bi00643a031. [DOI] [PubMed] [Google Scholar]
- Gagnon J., Christie D. L. Amino acid sequence of the Bb fragment from human complement Factor B. Alignment of the cyanogen bromide-cleavage peptides. Biochem J. 1983 Jan 1;209(1):51–60. doi: 10.1042/bj2090051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. M., Gagnon J., Reid K. B. Factor D of the alternative pathway of human complement. Purification, alignment and N-terminal amino acid sequences of the major cyanogen bromide fragments, and localization of the serine residue at the active site. Biochem J. 1980 Jun 1;187(3):863–874. doi: 10.1042/bj1870863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr M. A. Limited proteolysis of complement components C2 and factor B. Structural analogy and limited sequence homology. Biochem J. 1979 Dec 1;183(3):615–622. doi: 10.1042/bj1830615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr M. A., Porter R. R. The purification and properties of the second component of human complement. Biochem J. 1978 Apr 1;171(1):99–107. doi: 10.1042/bj1710099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesavre P. H., Hugli T. E., Esser A. F., Müller-Eberhard H. J. The alternative pathway C3/C5 convertase: chemical basis of factor B activation. J Immunol. 1979 Aug;123(2):529–534. [PubMed] [Google Scholar]
- Lewis R. V., Fallon A., Stein S., Gibson K. D., Udenfriend S. Supports for reverse-phase high-performance liquid chromatography of large proteins. Anal Biochem. 1980 May 1;104(1):153–159. doi: 10.1016/0003-2697(80)90291-2. [DOI] [PubMed] [Google Scholar]
- Mole J. E., Niemann M. A. Structural evidence that complement factor B constitutes a novel class of serine protease. J Biol Chem. 1980 Sep 25;255(18):8472–8476. [PubMed] [Google Scholar]
- Segal D. M., Powers J. C., Cohen G. H., Davies D. R., Wilcox P. E. Substrate binding site in bovine chymotrypsin A-gamma. A crystallographic study using peptide chloromethyl ketones as site-specific inhibitors. Biochemistry. 1971 Sep 28;10(20):3728–3738. doi: 10.1021/bi00796a014. [DOI] [PubMed] [Google Scholar]
- Stroud R. M., Kay L. M., Dickerson R. E. The crystal and molecular structure of DIP-inhibited bovine trypsin at2.7Angstrom resolution. Cold Spring Harb Symp Quant Biol. 1972;36:125–140. doi: 10.1101/sqb.1972.036.01.018. [DOI] [PubMed] [Google Scholar]
- Tack B. F., Morris S. C., Prahl J. W. Third component of human complement: structural analysis of the polypeptide chains of C3 and C3b. Biochemistry. 1979 Apr 17;18(8):1497–1503. doi: 10.1021/bi00575a017. [DOI] [PubMed] [Google Scholar]
- Woodbury R. G., Katunuma N., Kobayashi K., Titani K., Neurath H., Anderson W. F., Matthews B. W. Covalent structure of a group-specific protease from rat small intestine. Appendix: crystallographic data for a group specific protease from rat intestine. Biochemistry. 1978 Mar 7;17(5):811–819. doi: 10.1021/bi00598a010. [DOI] [PubMed] [Google Scholar]


