Abstract
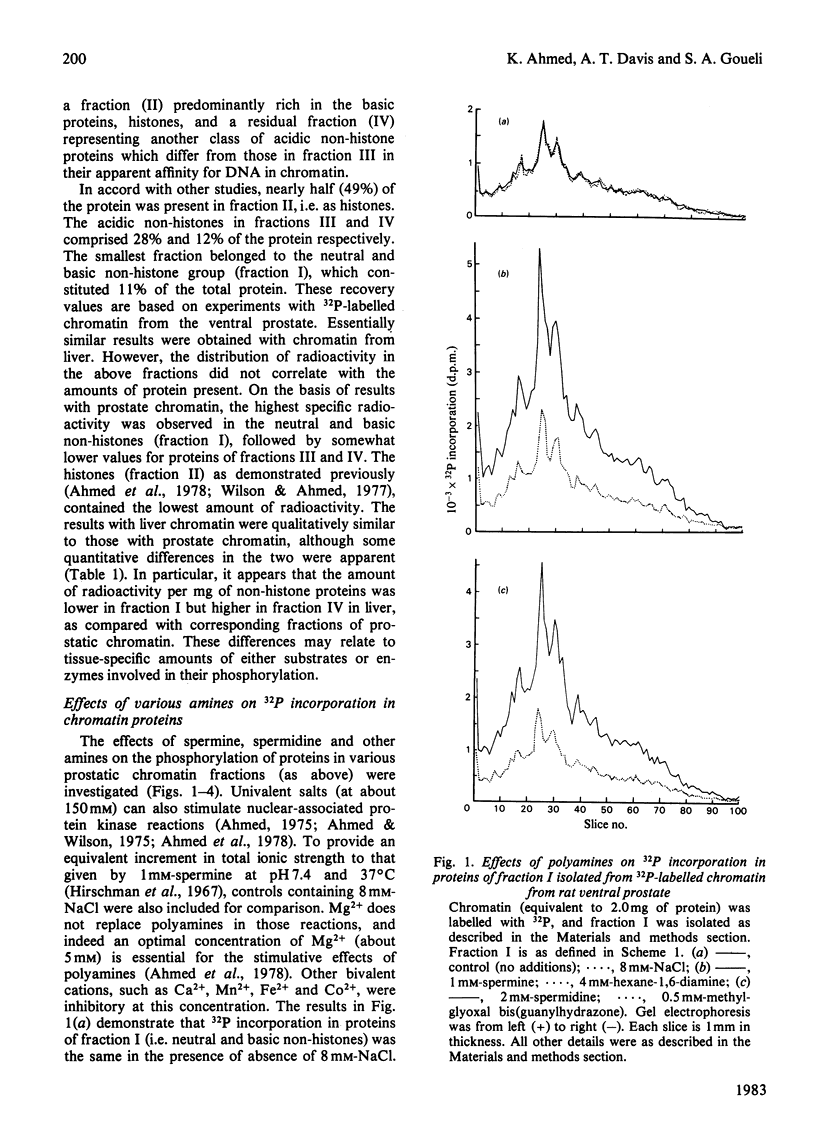
Studies are presented on the nature of chromatin-associated phosphoproteins whose phosphorylation is influenced by polyamines. After labelling with 32P, chromatin-associated proteins were separated into four fractions. Fraction I comprised neutral and basic non-histone phosphoproteins, including high-mobility-group non-histones; fraction II consisted mostly of histones; fraction III consisted of a class of (salt-soluble) acidic non-histone phosphoproteins; and fraction IV consisted of residual (salt-insoluble) acidic non-histone phosphoproteins. The average relative distribution of protein in the four fractions (I-IV) was about 1:4:2:1 for both liver and prostate. However, tissue-dependent differences were observed in the incorporation of 32P in various protein fractions. In the presence of polyamines (e.g. 1 mM-spermine or 2 mM-spermidine) maximal stimulation of phosphorylation was observed in non-histone proteins of fraction I (160-180%), followed by that in non-histone proteins of fraction III (80-110%). The phosphorylation of residual non-histone proteins in fraction IV, and the small extent of phosphorylation of histones in fraction II, remained unaltered in the presence of polyamines. Thus polyamines do not stimulate the phosphorylation of all non-histone proteins; their stimulative effect is most prominent in the phosphorylation of neutral and basic non-histone proteins and a class of salt-soluble acidic non-histone proteins. In accord with our hypothesis, these differential effects of polyamines on phosphorylation of endogenous non-histone proteins may relate to the conformation of these substrates rather than to endogenous kinases.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed K., Davis A. T., Goueli S. A., Wilson M. J. Phosphorylation of a nonhistone protein fraction which coextracts with the high-mobility-group proteins of chromatin. Biochem Biophys Res Commun. 1980 Sep 16;96(1):326–332. doi: 10.1016/0006-291x(80)91218-8. [DOI] [PubMed] [Google Scholar]
- Ahmed K., Ishida H. Effect of testosterone on nuclear phosphoproteins of rat ventral prostate. Mol Pharmacol. 1971 May;7(3):323–327. [PubMed] [Google Scholar]
- Ahmed K., Wilson M. J. Chromatin-associated protein phosphokinases of rat ventral prostate. Characteristics and effects of androgenic status. J Biol Chem. 1975 Mar 25;250(6):2370–2375. [PubMed] [Google Scholar]
- Ahmed K., Wilson M. J., Goueli S. A., Williams-Ashman H. G. Effects of polyamines on prostatic chromatin- and non-histone-protein-associated protein kinase reactions. Biochem J. 1978 Dec 15;176(3):739–750. doi: 10.1042/bj1760739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmar V. J., Daniels G. R., Kuehn G. D. Polyamine stimulation of phosphorylation of nonhistone acidic protein in nuclei and nucleoli from Physarum polycephalum. Eur J Biochem. 1978 Sep 15;90(1):29–37. doi: 10.1111/j.1432-1033.1978.tb12571.x. [DOI] [PubMed] [Google Scholar]
- Blakesley R. W., Boezi J. A. A new staining technique for proteins in polyacrylamide gels using coomassie brilliant blue G250. Anal Biochem. 1977 Oct;82(2):580–582. doi: 10.1016/0003-2697(77)90197-x. [DOI] [PubMed] [Google Scholar]
- Farron-Furstenthal F., Lightholder J. R. Effects of polyamines and histones on the phosphorylation of non-histone proteins in isolated rat liver nuclei. Biochem Biophys Res Commun. 1978 Jul 14;83(1):94–100. doi: 10.1016/0006-291x(78)90402-3. [DOI] [PubMed] [Google Scholar]
- Goodwin G. H., Nicolas R. H., Johns E. W. An improved large scale fractionation of high mobility group non-histone chromatin proteins. Biochim Biophys Acta. 1975 Oct 20;405(2):280–291. doi: 10.1016/0005-2795(75)90094-x. [DOI] [PubMed] [Google Scholar]
- Goueli S. A., Steer R. C., Wilson M. J., Ahmed K. Partial purification and differential androgen sensitivity of protein phosphokinases from nuclei of rat ventral prostate. Eur J Biochem. 1980 Dec;113(1):45–51. doi: 10.1111/j.1432-1033.1980.tb06137.x. [DOI] [PubMed] [Google Scholar]
- Hirschman S., Leng M., Felsenfield G. Interaction of spermine and DNA. Biopolymers. 1967 Feb;5(2):227–233. doi: 10.1002/bip.1967.360050209. [DOI] [PubMed] [Google Scholar]
- Imai H., Shimoyama M., Yamamoto S., Tanigawa Y., Ueda I. Effect of polyamines on phosphorylation of non-histone chromatin proteins from hog liver. Biochem Biophys Res Commun. 1975 Sep 16;66(2):856–862. doi: 10.1016/0006-291x(75)90588-4. [DOI] [PubMed] [Google Scholar]
- Ishida H., Ahmed K. Studies on chromatin-associated protein phosphokinase of submandibular gland from isoproterenol-treated rats. Exp Cell Res. 1974 Mar 15;84(1):127–136. doi: 10.1016/0014-4827(74)90388-7. [DOI] [PubMed] [Google Scholar]
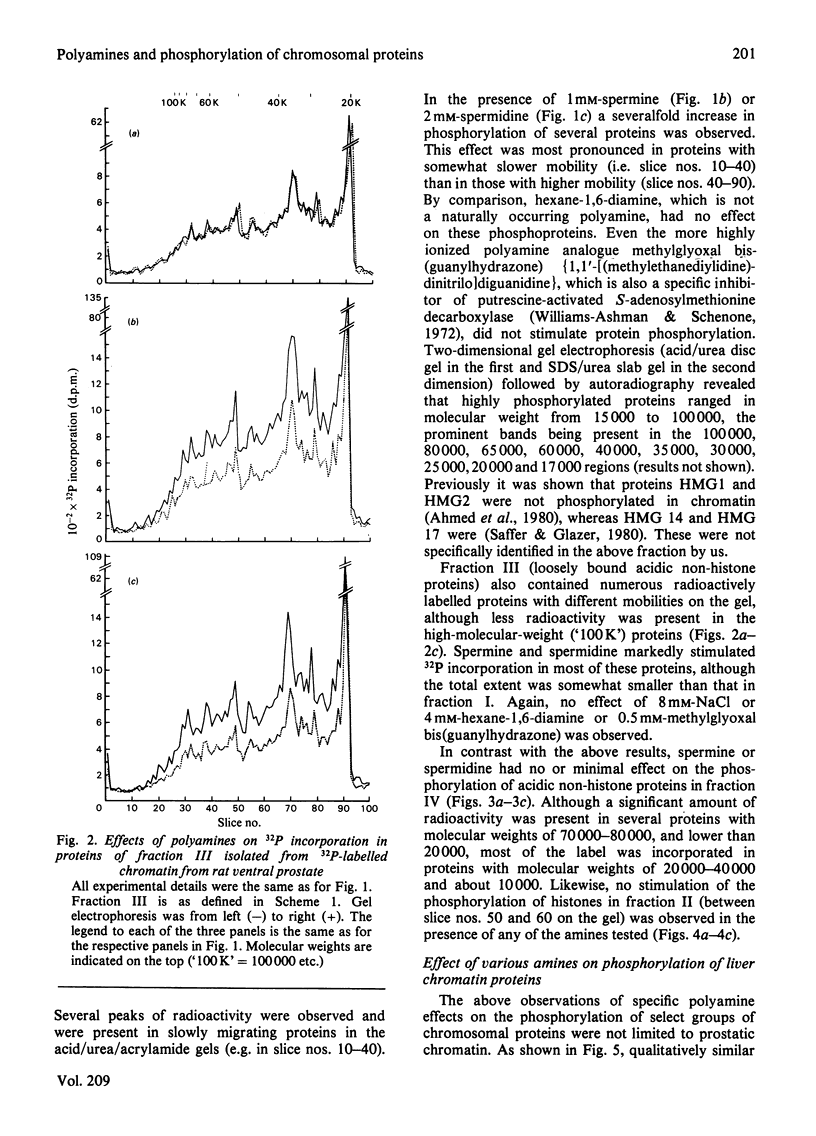
- Kaplowitz P. B., Platz R. D., Kleinsmith L. J. Nuclear phosphoproteins. 3. Increase in phosphory- lation during histone-phosphoprotein interaction. Biochim Biophys Acta. 1971 Mar 23;229(3):739–748. [PubMed] [Google Scholar]
- Kish V. M., Kleinsmith L. J. Nuclear protein kinases. Evidence for their heterogeneity, tissue specificity, substrate specificities, and differential responses to cyclic adenosine 3':5'-monophosphate. J Biol Chem. 1974 Feb 10;249(3):750–760. [PubMed] [Google Scholar]
- Klyzsejko-Stefanowicz L., Chiu J. F., Tsai Y. H., Hnilica L. S. Acceptor proteins in rat androgenic tissue chromatin. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1954–1958. doi: 10.1073/pnas.73.6.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S., Simpson R. T., Sober H. A. Fractionation of chromatin components. Biochemistry. 1972 Apr 25;11(9):1547–1554. [PubMed] [Google Scholar]
- Macgillivray A. J., Paul J., Threlfall G. Transcriptional regulation in eukaryotic cells. Adv Cancer Res. 1972;15:93–162. doi: 10.1016/s0065-230x(08)60373-5. [DOI] [PubMed] [Google Scholar]
- Mainwaring W. I., Symes E. K., Higgins S. J. Nuclear components responsible for the retention of steroid--receptor complexes, especially from the standpoint of the specifcity of hormonal responses. Biochem J. 1976 Apr 15;156(1):129–141. doi: 10.1042/bj1560129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L. M., Wang T. Y. The role of the androgen-binding nonhistone proteins in the transcription of prostatic chromatin. J Steroid Biochem. 1976 Apr;7(4):267–273. doi: 10.1016/0022-4731(76)90126-6. [DOI] [PubMed] [Google Scholar]
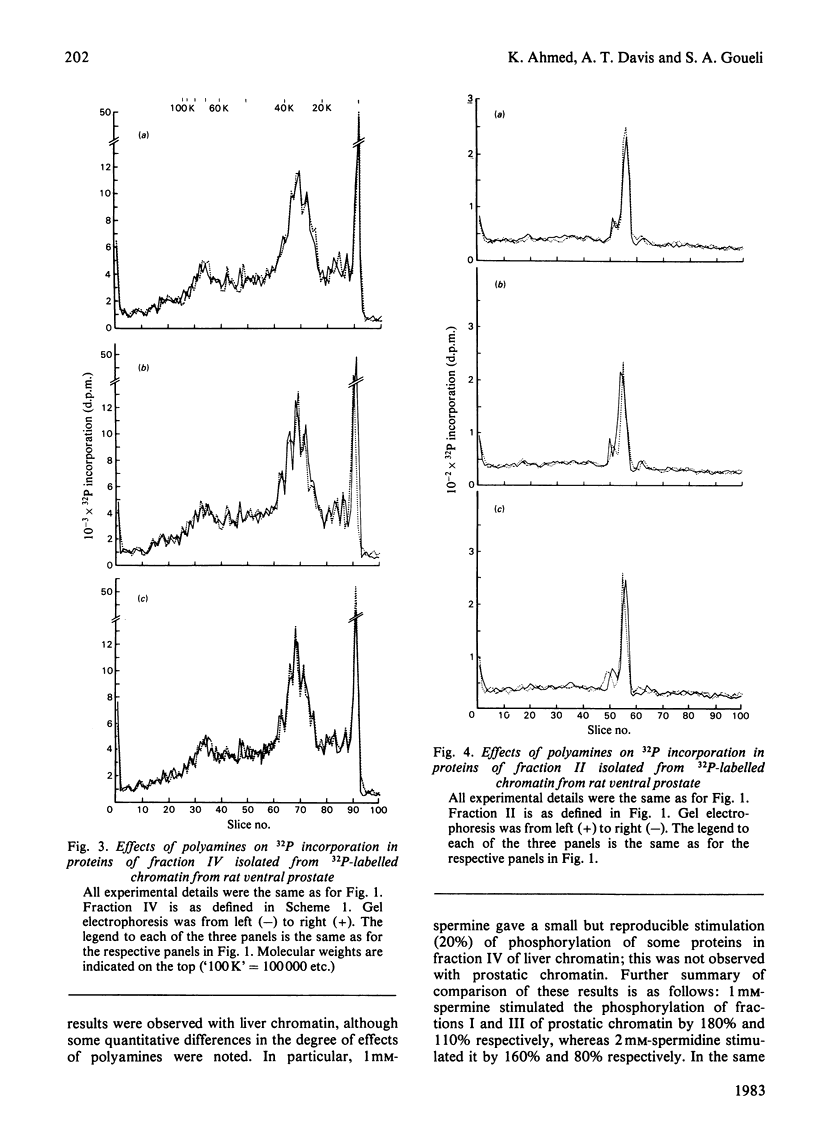
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Lockwood D. H., Williams-Ashman H. G. Concentrations of putrescine and polyamines and their enzymic synthesis during androgen-induced prostatic growth. Biochem J. 1970 Mar;117(1):17–31. doi: 10.1042/bj1170017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffer J. D., Glazer R. I. The phosphorylation of high mobility group proteins 14 and 17 from Ehrlich ascites and L1210 in vitro. Biochem Biophys Res Commun. 1980 Apr 29;93(4):1280–1285. doi: 10.1016/0006-291x(80)90628-2. [DOI] [PubMed] [Google Scholar]
- Scalabrino G., Ferioli M. E. Polyamines in mammalian tumors. Part I. Adv Cancer Res. 1981;35:151–268. doi: 10.1016/s0065-230x(08)60911-2. [DOI] [PubMed] [Google Scholar]
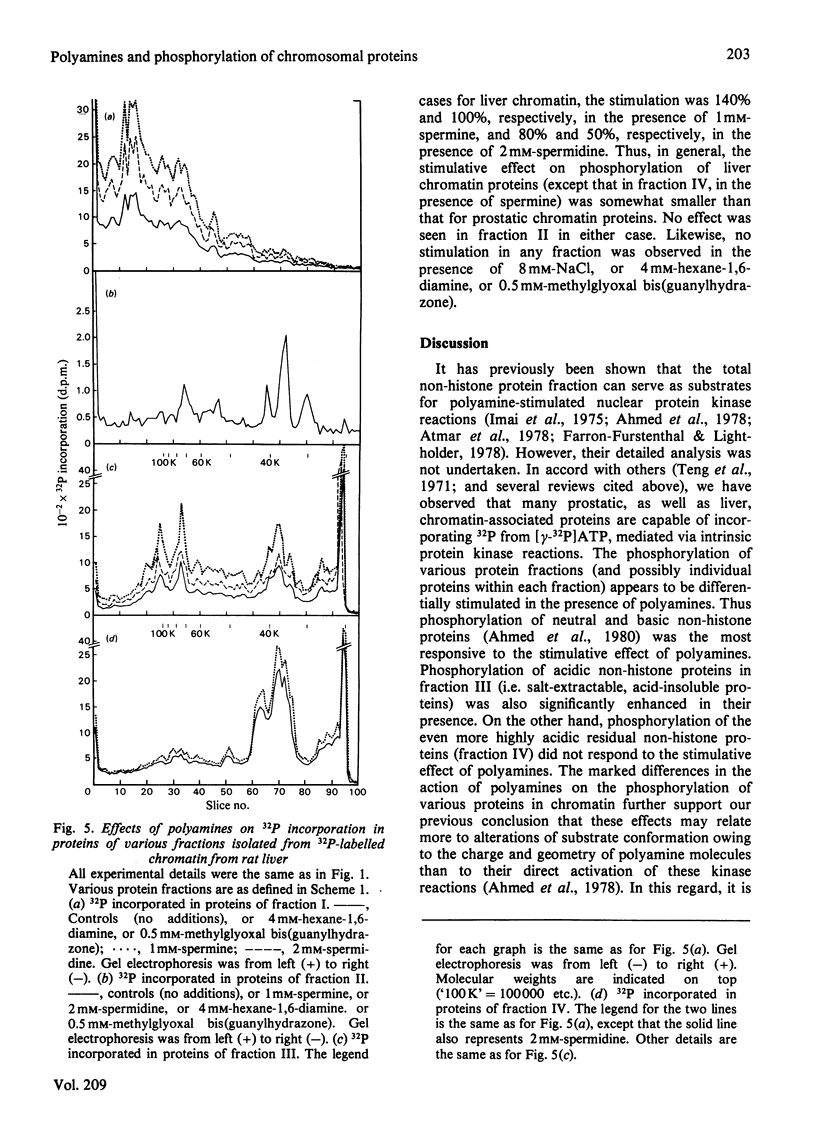
- Stein G. S., Spelsberg T. C., Kleinsmith L. J. Nonhistone chromosomal proteins and gene regulation. Science. 1974 Mar 1;183(4127):817–824. doi: 10.1126/science.183.4127.817. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Tymoczko J. L., Liao S. Retention of an androgen-protein complex by nuclear chromatin aggregates: heat-labile factors. Biochim Biophys Acta. 1971 Dec 21;252(3):607–611. doi: 10.1016/0304-4165(71)90168-1. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Canellakis Z. N. Transglutaminase-mediated covalent attachment of polyamines to proteins: mechanisms and potential physiological significance. Physiol Chem Phys. 1980;12(5):457–472. [PubMed] [Google Scholar]
- Williams-Ashman H. G., Corti A., Tadolini B. On the development of specific inhibitors of animal polyamine biosynthetic enzymes. Ital J Biochem. 1976 Jan-Feb;25(1):5–32. [PubMed] [Google Scholar]
- Williams-Ashman H. G., Pegg A. E., Lockwood D. H. Mechanisms and regulation of polyamine and putrescine biosynthesis in male genital glands and other tissues of mammals. Adv Enzyme Regul. 1969;7:291–323. doi: 10.1016/0065-2571(69)90024-7. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Schenone A. Methyl glyoxal bis(guanylhydrazone) as a potent inhibitor of mammalian and yeast S-adenosylmethionine decarboxylases. Biochem Biophys Res Commun. 1972 Jan 14;46(1):288–295. doi: 10.1016/0006-291x(72)90661-4. [DOI] [PubMed] [Google Scholar]
- Wilson M. J., Ahmed K. Enzymic characteristics and effects of testosterone treatment on nucleolar and chromatin-associated histone phosphokinase activity of rat ventral prostate. Exp Cell Res. 1977 Apr;106(1):151–157. doi: 10.1016/0014-4827(77)90251-8. [DOI] [PubMed] [Google Scholar]


