Abstract
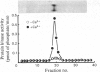
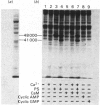
A phospholipid-sensitive Ca2+-dependent protein kinase was purified to homogeneity, for the first time, from extracts of pig spleen, employing the steps of DEAE-cellulose, octyl-agarose, Sephacryl S-200 and phosphatidylserine-Affigel 10 affinity chromatographies. The purified enzyme appeared as a single protein band on both analytical (non-denaturing) and sodium dodecyl sulphate/polyacrylamide-gel electrophoresis, having a minimum mol.wt. of 68 000 +/- 200. The molecular weight of the enzyme was also determined to be 74 500 +/- 4600 by gel filtration and 80 000 based on its sedimentation coefficient (5.52 S) and Stokes radius (3.52 +/- 0.09 nm), indicating that the enzyme was a monomeric protein. The frictional ratio (f/f0) of the enzyme was 1.24, indicating it was non-globular in shape. The enzyme had a pI of 5.3, and a pH optimum of 6.5 for its reaction. Amino acid analysis indicated that the enzyme apparently was not similar to myosin light-chain kinase (a calmodulin-sensitive species of Ca2+-dependent protein kinase) or cyclic AMP-dependent and cyclic GMP-dependent protein kinases. The enzyme had an apparent Km for ATP of 7.5 microns. Histone H1 and myelin basic protein were effective substrates for the enzyme, with apparent Km values of 0.3 and 0.2 microns, and Vmax, values of 0.06 and 0.09 mumol/min per mg of enzyme respectively. The enzyme activity was dependent on both phosphatidylserine (apparent Ka = 6.25 micrograms/ml) and Ca2+ (apparent Ka = 160 microns). Calmodulin was unable to substitute for the phospholipid as a cofactor, nor was it a subunit of the enzyme. Sr2+ and Ba2+ could partially mimic Ca2+ to activate the enzyme in the presence of phosphatidylserine. An endogenous substrate protein (mol.wt. 41 000) for the enzyme was found in the total, solubilized fraction of pig spleen. Monoclonal antibodies against the enzyme interacted similarly with the homogeneous and impure enzyme; the antibodies, however, did not bind to cyclic nucleotide-dependent protein kinases.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Klee C. B. Purification and characterization of smooth muscle myosin light chain kinase. J Biol Chem. 1981 Jul 25;256(14):7501–7509. [PubMed] [Google Scholar]
- Benson J. V., Jr, Patterson J. A. Accelerated chromatographic analysis of amino acids commonly found in physiological fluids on a spherical resin of specific design. Anal Biochem. 1965 Nov;13(2):265–280. doi: 10.1016/0003-2697(65)90196-x. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. The interaction of cyclic nucleotides and calcium in the control of cellular activity. Adv Cyclic Nucleotide Res. 1975;6:1–98. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Chou F. C., Chou C. H., Shapira R., Kibler R. F. Basis of microheterogeneity of myelin basic protein. J Biol Chem. 1976 May 10;251(9):2671–2679. [PubMed] [Google Scholar]
- Gefter M. L., Margulies D. H., Scharff M. D. A simple method for polyethylene glycol-promoted hybridization of mouse myeloma cells. Somatic Cell Genet. 1977 Mar;3(2):231–236. doi: 10.1007/BF01551818. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Antibody production by hybridomas. J Immunol Methods. 1980;39(4):285–308. doi: 10.1016/0022-1759(80)90230-6. [DOI] [PubMed] [Google Scholar]
- Helfman D. M., Shoji M., Kuo J. F. Purification to homogeneity and general properties of a novel phosphodiesterase hydrolyzing cyclic CMP and cyclic AMP. J Biol Chem. 1981 Jun 25;256(12):6327–6334. [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Nishizuka Y. Cooperative roles of various membrane phospholipids in the activation of calcium-activated, phospholipid-dependent protein kinase. J Biol Chem. 1981 Jul 25;256(14):7146–7149. [PubMed] [Google Scholar]
- Katoh N., Kuo J. F. Subcellular distribution of phospholipid-sensitive calcium-dependent protein kinase in guinea pig heart, spleen and cerebral cortex, and inhibition of the enzyme by Triton X-100. Biochem Biophys Res Commun. 1982 May 31;106(2):590–595. doi: 10.1016/0006-291x(82)91151-2. [DOI] [PubMed] [Google Scholar]
- Katoh N., Wise B. C., Wrenn R. W., Kuo J. F. Inhibition by adriamycin of calmodulin-sensitive and phospholipid-sensitive calcium-dependent phosphorylation of endogenous proteins from heart. Biochem J. 1981 Jul 15;198(1):199–205. doi: 10.1042/bj1980199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh N., Wrenn R. W., Wise B. C., Shoji M., Kuo J. F. Substrate proteins for calmodulin-sensitive and phospholipid-sensitive Ca2+-dependent protein kinases in heart, and inhibition of their phosphorylation by palmitoylcarnitine. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4813–4817. doi: 10.1073/pnas.78.8.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara Y., Takai Y., Minakuchi R., Sano K., Nishizuka Y. Phospholipid turnover as a possible transmembrane signal for protein phosphorylation during human platelet activation by thrombin. Biochem Biophys Res Commun. 1980 Nov 17;97(1):309–317. doi: 10.1016/s0006-291x(80)80169-0. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Kuo J. F., Andersson R. G., Wise B. C., Mackerlova L., Salomonsson I., Brackett N. L., Katoh N., Shoji M., Wrenn R. W. Calcium-dependent protein kinase: widespread occurrence in various tissues and phyla of the animal kingdom and comparison of effects of phospholipid, calmodulin, and trifluoperazine. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7039–7043. doi: 10.1073/pnas.77.12.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln T. M., Corbin J. D. Adenosine 3':5'-cyclic monophosphate- and guanosine 3':5'-cyclic monophosphate-dependent protein kinases: possible homologous proteins. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3239–3243. doi: 10.1073/pnas.74.8.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Randolph D. H., Kibler R. F., Fritz R. B. Solid-phase radioimmunoassay for detection of antibodies to myelin basic protein. J Immunol Methods. 1977;18(3-4):215–224. doi: 10.1016/0022-1759(77)90175-2. [DOI] [PubMed] [Google Scholar]
- Schatzman R. C., Wise B. C., Kuo J. F. Phospholipid-sensitive calcium-dependent protein kinase: inhibition by antipsychotic drugs. Biochem Biophys Res Commun. 1981 Feb 12;98(3):669–676. doi: 10.1016/0006-291x(81)91166-9. [DOI] [PubMed] [Google Scholar]
- Shoji M., Patrick J. G., Davis C. W., Kuo J. F. Guanosine cyclic monophosphate-dependent protein kinase from foetal calf heart. Purification, general properties and catalytic subunit. Biochem J. 1977 Feb 1;161(2):213–221. doi: 10.1042/bj1610213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Iwasa Y., Kawahara Y., Mori T., Nishizuka Y. Calcium-dependent activation of a multifunctional protein kinase by membrane phospholipids. J Biol Chem. 1979 May 25;254(10):3692–3695. [PubMed] [Google Scholar]
- Wang J. H., Waisman D. M. Calmodulin and its role in the second-messenger system. Curr Top Cell Regul. 1979;15:47–107. doi: 10.1016/b978-0-12-152815-7.50006-5. [DOI] [PubMed] [Google Scholar]
- Wise B. C., Glass D. B., Chou C. H., Raynor R. L., Katoh N., Schatzman R. C., Turner R. S., Kibler R. F., Kuo J. F. Phospholipid-sensitive Ca2+-dependent protein kinase from heart. II. Substrate specificity and inhibition by various agents. J Biol Chem. 1982 Jul 25;257(14):8489–8495. [PubMed] [Google Scholar]
- Wise B. C., Raynor R. L., Kuo J. F. Phospholipid-sensitive Ca2+-dependent protein kinase from heart. I. Purification and general properties. J Biol Chem. 1982 Jul 25;257(14):8481–8488. [PubMed] [Google Scholar]
- Wrenn R. W., Katoh N., Wise B. C., Kuo J. F. Stimulation by phosphatidylserine and calmodulin of calcium-dependent phosphorylation of endogenous proteins from cerebral cortex. J Biol Chem. 1980 Dec 25;255(24):12042–12046. [PubMed] [Google Scholar]