Abstract
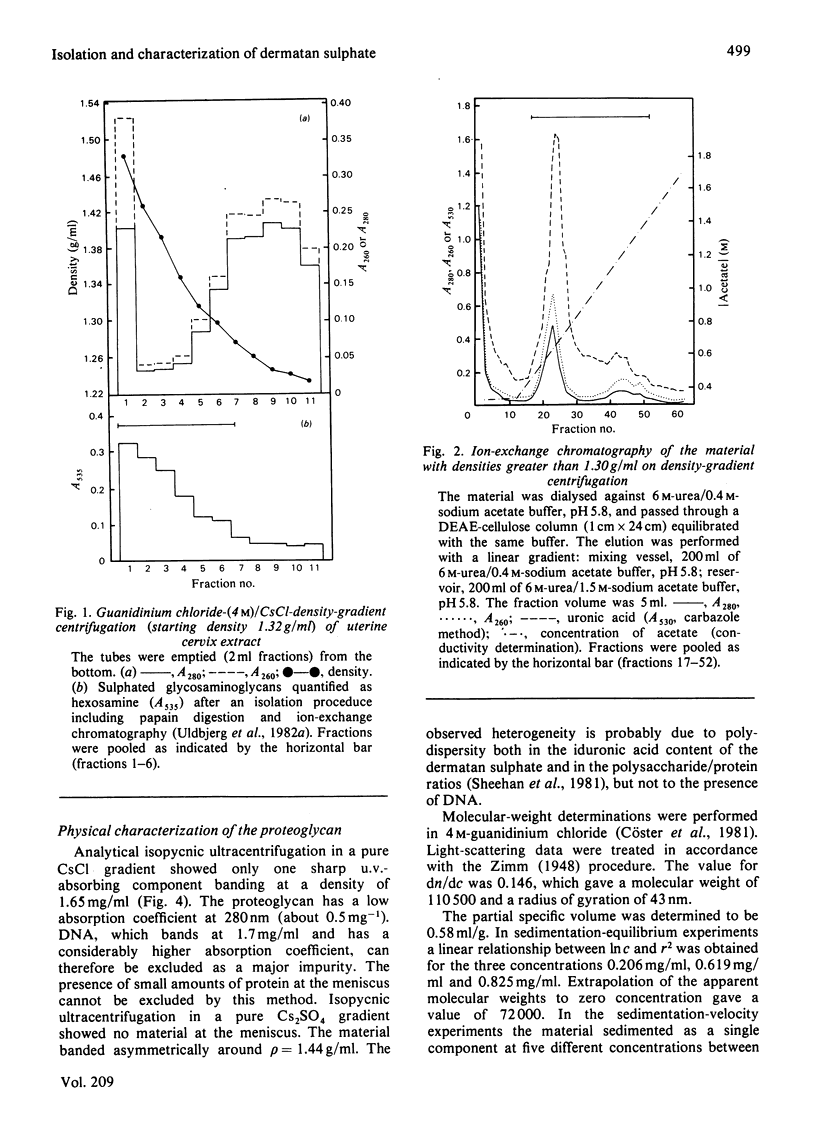
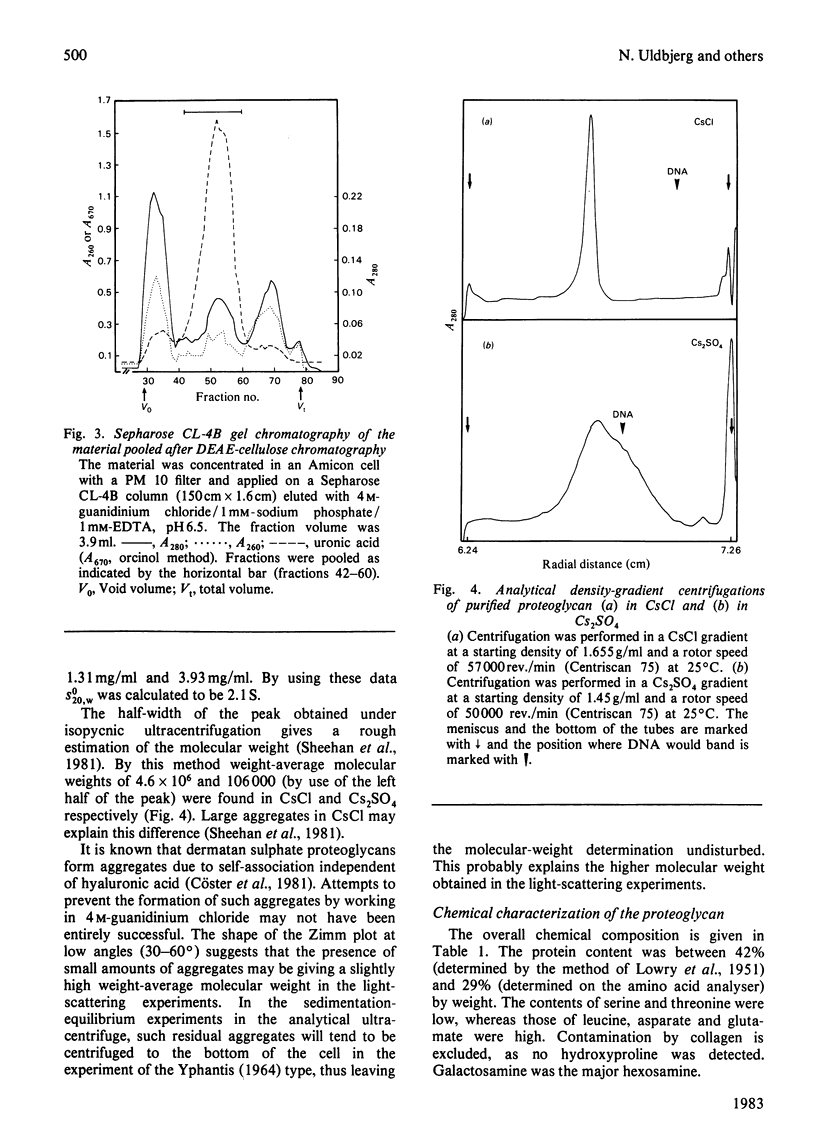
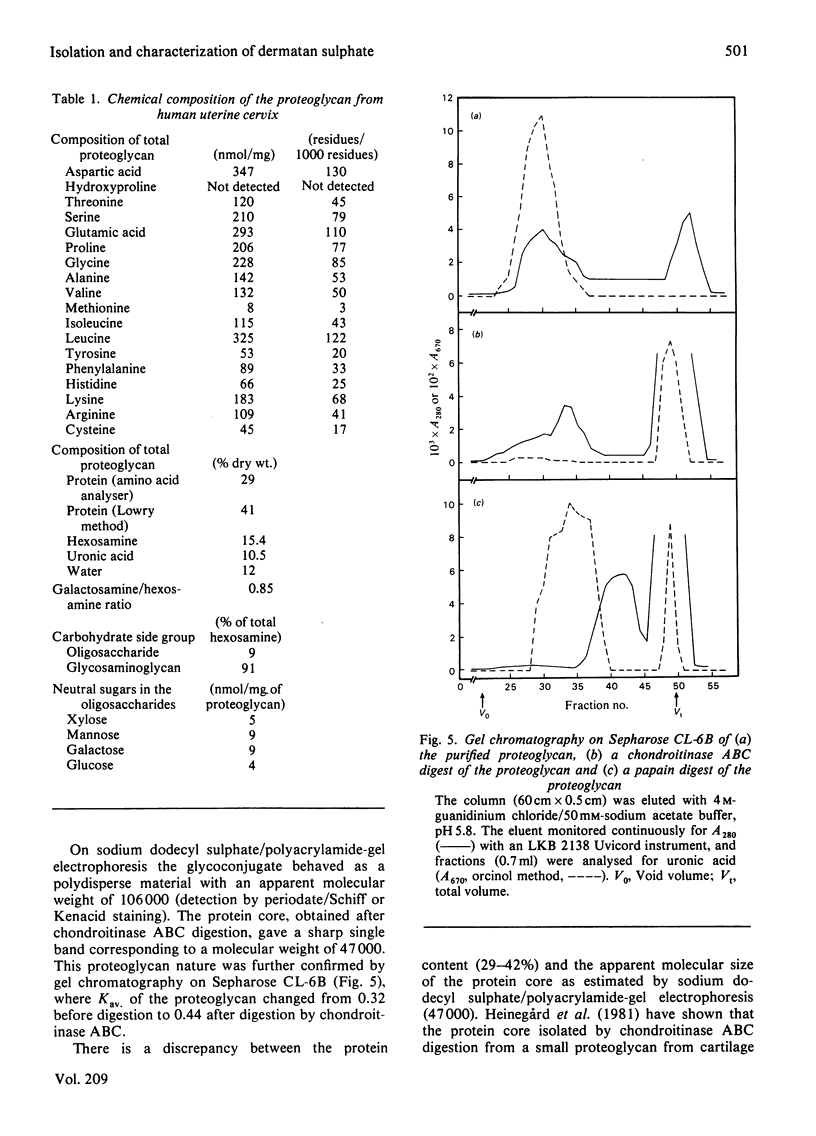
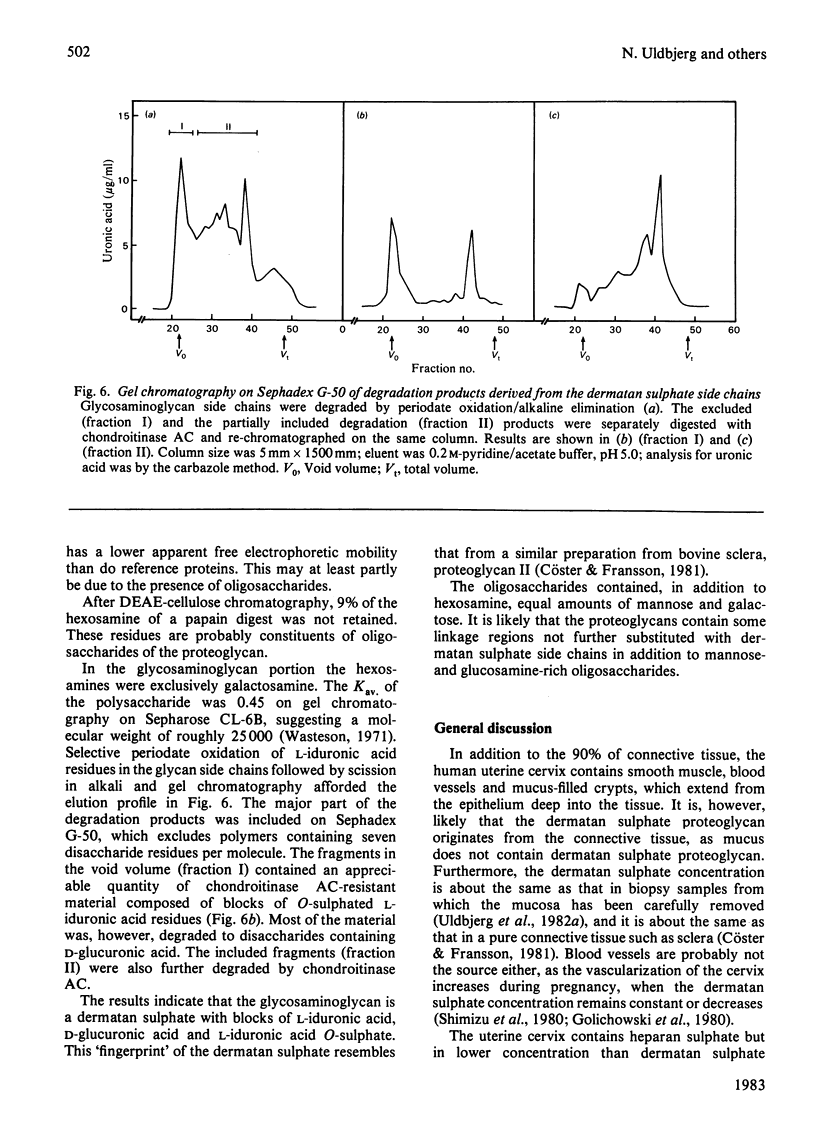
Proteoglycans were extracted from human uterine cervix with 4 M-guanidinium chloride in the presence of proteinase inhibitors. They were purified by density-gradient centrifugation in 4 M-guanidinium chloride/CsCl (starting density 1.32 g/ml) followed by DEAE-cellulose and Sepharose chromatography. Only one polydisperse proteoglycan was found. s020,w was 2.1S and the weight-average molecular weight was 73 000 (sedimentation-equilibrium centrifugation) to 110 500 (light-scattering). The core protein was monodisperse, with an apparent molecular weight of 47 000. The proteoglycan contained about 30% protein and probably two or three glycosaminoglycan side chains per molecule. High contents of aspartate, glutamate and leucine were found. The glycan moiety of the proteoglycan was exclusively dermatan sulphate, with a co-polymeric structure with approximately equal quantities of iduronic acid- and glucuronic acid-containing disaccharides.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTONOPOULOS C. A., GARDELL S., SZIRMAI J. A., DETYSSONSK E. R. DETERMINATION OF GLYCOSAMINOGLYCANS (MUCOPOLYSACCHARIDES) FROM TISSUE ON THE MICROGRAM SCALE. Biochim Biophys Acta. 1964 Mar 2;83:1–19. doi: 10.1016/0926-6526(64)90045-x. [DOI] [PubMed] [Google Scholar]
- Axelsson I., Heinegård D. Fractionation of proteoglycans from bovine corneal stroma. Biochem J. 1975 Mar;145(3):491–500. doi: 10.1042/bj1450491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Carlstedt I., Cöster L., Malmström A. Isolation and characterization of dermatan sulphate and heparan sulphate proteoglycans from fibroblast culture. Biochem J. 1981 Jul 1;197(1):217–225. doi: 10.1042/bj1970217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cöster L., Fransson L. A. Isolation and characterization of dermatan sulphate proteoglycans from bovine sclera. Biochem J. 1981 Jan 1;193(1):143–153. doi: 10.1042/bj1930143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cöster L., Fransson L. A., Sheehan J., Nieduszynski I. A., Phelps C. F. Self-association of dermatan sulphate proteoglycans from bovine sclera. Biochem J. 1981 Aug 1;197(2):483–490. doi: 10.1042/bj1970483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman A., Ulmsten U., Bányai J., Wingerup L., Uldbjerg N. Evidence for a local effect of intracervical prostaglandin E2-gel. Am J Obstet Gynecol. 1982 Aug 1;143(7):756–760. doi: 10.1016/0002-9378(82)90005-9. [DOI] [PubMed] [Google Scholar]
- Fransson L. A., Cöster L. Interaction between dermatan sulphate chains. II. Structural studies on aggregating glycan chains and oligosaccharides with affinity for dermatan sulphate-substituted agarose. Biochim Biophys Acta. 1979 Jan 4;582(1):132–144. doi: 10.1016/0304-4165(79)90296-4. [DOI] [PubMed] [Google Scholar]
- Golichowski A. M., King S. R., Mascaro K. Pregnancy-related changes in rat cervical glycosaminoglycans. Biochem J. 1980 Oct 15;192(1):1–8. doi: 10.1042/bj1920001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinegård D., Paulsson M., Inerot S., Carlström C. A novel low-molecular weight chondroitin sulphate proteoglycan isolated from cartilage. Biochem J. 1981 Aug 1;197(2):355–366. doi: 10.1042/bj1970355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hök M., Lindahl U., Iverius P. H. Distribution of sulphate and iduronic acid residues in heparin and heparan sulphate. Biochem J. 1974 Jan;137(1):33–43. doi: 10.1042/bj1370033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleissl H. P., van der Rest M., Naftolin F., Glorieux F. H., de Leon A. Collagen changes in the human uterine cervix at parturition. Am J Obstet Gynecol. 1978 Apr 1;130(7):748–753. doi: 10.1016/0002-9378(78)90003-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Rorie D. K., Newton M. Histologic and chemical studies of the smooth muscle in the human cervix and uterus. Am J Obstet Gynecol. 1967 Oct 15;99(4):466–469. doi: 10.1016/0002-9378(67)90292-x. [DOI] [PubMed] [Google Scholar]
- Sheehan J. K., Carlstedt I., Cöster L., Malmström A., Fransson L. A. Isopycnic-centrifugation studies in caesium chloride and in caesium sulphate on dermatan sulphate proteoglycans from bovine sclera. Biochem J. 1981 Dec 1;199(3):581–589. doi: 10.1042/bj1990581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Endo M., Yosizawa Z. Glycoconjugates (glycosaminoglycans and glycoproteins) and glycogen in the human cervix uteri. Tohoku J Exp Med. 1980 Jul;131(3):289–299. doi: 10.1620/tjem.131.289. [DOI] [PubMed] [Google Scholar]
- Wasteson A. A method for the determination of the molecular weight and molecular-weight distribution of chondroitin sulphate. J Chromatogr. 1971 Jul 8;59(1):87–97. doi: 10.1016/s0021-9673(01)80009-1. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]


