Abstract
The alpha-helix-rich particle of Mr 50 200, derived by limited alpha-chymotryptic digestion of the solubilized microfibrillar proteins from wool alpha-keratin, consists mainly of polypeptide-chain segments of Mr 12 500 (fraction ChC) and 25 000 (fraction ChB). The 12 500-Mr segments are of two types (I and II), which are derived from different polypeptide chains of the microfibrillar complex. Each of these type-I and type-II segments partially self-associates in benign solvents to form either dimers or tetramers. When mixed, the two segments show changes in physical properties (alpha-helix content, difference spectra and molecular weight) indicative of complex-formation. The maximum changes occur when the two segments are mixed in an equimolar ratio. Complexes isolated after rapid dialysis of mixtures from 8 M-urea solution were examined by various methods. A tetrameric structure is the main product formed in all cases, and the maximum amount of tetramer is obtained from equimolar mixtures of the type-I and type-II polypeptides. When urea is removed by dialysis from the unfractionated 12 500-Mr segments (fraction ChC) or from the alpha-helix-rich particle itself, a similar complex of Mr 50 000 is formed. The physical properties of these reconstituted entities (alpha-helix content, molecular weight, thermal stability and exposure of tyrosine residues) are similar to those of the original alpha-helix-rich particle. Cross-linking experiments with dimethyl suberimidate are in agreement with a four-chain complex for the reassembled structures. A pair of double-stranded alpha-helices is proposed for the particle, and is considered to be an integral part of the microfibrillar complex in wool alpha-keratin.
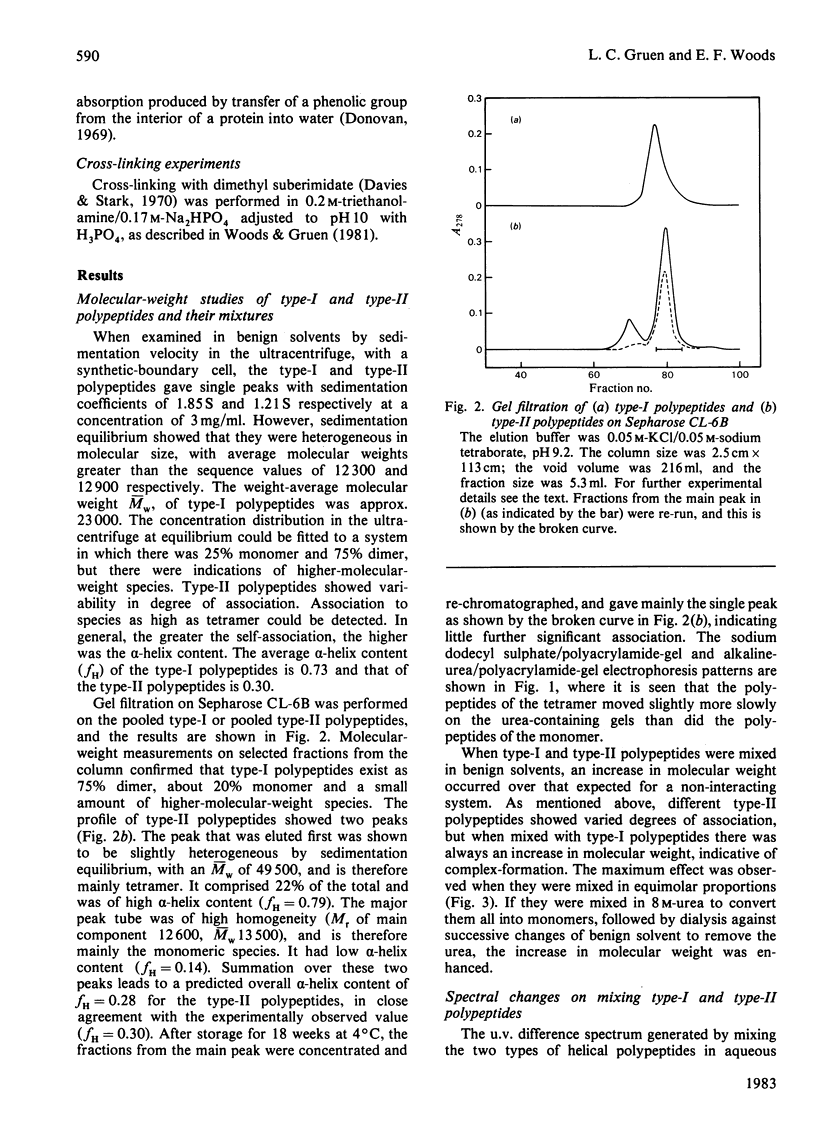
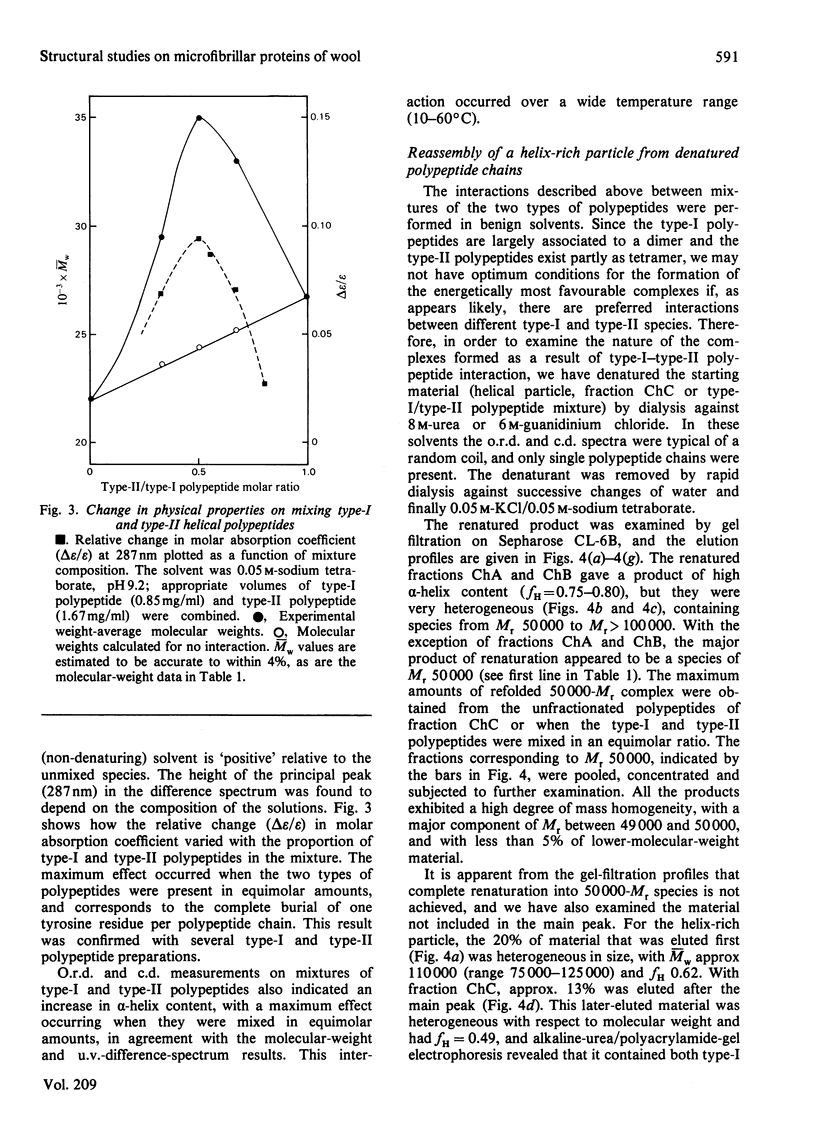
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmadi B., Speakman P. T. Suberimidate crosslinking shows that a rod-shaped, low cystine, high helix protein prepared by limited proteolysis of reduced wool has four protein chains. FEBS Lett. 1978 Oct 15;94(2):365–367. doi: 10.1016/0014-5793(78)80978-8. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Chau K. H. Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry. 1974 Jul 30;13(16):3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- Crewther W. G., Inglis A. S., McKern N. M. Amino acid sequences of alpha-helical segments from S-carboxymethylkerateine-A. Complete sequence of a type-II segment. Biochem J. 1978 Aug 1;173(2):365–371. doi: 10.1042/bj1730365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRASER R. D., MACRAE T. P., MILLER A. THE COILED-COIL MODEL OF ALPHA-KERATIN STRUCTURE. J Mol Biol. 1964 Oct;10:147–156. doi: 10.1016/s0022-2836(64)80034-6. [DOI] [PubMed] [Google Scholar]
- Fraser R. D., MacRae T. P., Miller A. X-ray diffraction patterns of alpha-fibrous proteins. J Mol Biol. 1965 Dec;14(2):432–442. doi: 10.1016/s0022-2836(65)80193-0. [DOI] [PubMed] [Google Scholar]
- Fraser R. D., MacRae T. P., Suzuki E. Structure of the alpha-keratin microfibril. J Mol Biol. 1976 Dec;108(2):435–452. doi: 10.1016/s0022-2836(76)80129-5. [DOI] [PubMed] [Google Scholar]
- Gough K. H., Inglis A. S., Crewther W. G. Amino acid sequences of alpha-helical segments from S-carbosymethylkerateine-A. Complete sequence of a type-I segment. Biochem J. 1978 Aug 1;173(2):373–385. doi: 10.1042/bj1730373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotay S. S., Speakman P. T. Three-chain merokeratin from wool may be a fragment of the microfibril component macromolecule. Nature. 1977 Jan 20;265(5591):274–276. doi: 10.1038/265274a0. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D. Coiled coil formation and sequence regularities in the helical regions of alpha-keratin. J Mol Biol. 1978 Sep 5;124(1):297–304. doi: 10.1016/0022-2836(78)90163-8. [DOI] [PubMed] [Google Scholar]
- PAULING L., COREY R. B. Compound helical configurations of polypeptide chains: structure of proteins of the alpha-keratin type. Nature. 1953 Jan 10;171(4341):59–61. doi: 10.1038/171059a0. [DOI] [PubMed] [Google Scholar]
- Parry D. A., Crewther W. G., Fraser R. D., MacRae T. P. Structure of alpha-keratin: structural implication of the amino acid sequences of the type I and type II chain segments. J Mol Biol. 1977 Jun 25;113(2):449–454. doi: 10.1016/0022-2836(77)90153-x. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W. The polypeptide composition of bovine epidermal alpha-keratin. Biochem J. 1975 Dec;151(3):603–614. doi: 10.1042/bj1510603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M. Structural features of the alpha-type filaments of the inner root sheath cells of the guinea pig hair follicle. Biochemistry. 1978 Nov 14;17(23):5045–5052. doi: 10.1021/bi00616a029. [DOI] [PubMed] [Google Scholar]
- Steinert P. M. Structure of the three-chain unit of the bovine epidermal keratin filament. J Mol Biol. 1978 Jul 25;123(1):49–70. doi: 10.1016/0022-2836(78)90376-5. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Zimmerman S. B., Starger J. M., Goldman R. D. Ten-nanometer filaments of hamster BHK-21 cells and epidermal keratin filaments have similar structures. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6098–6101. doi: 10.1073/pnas.75.12.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven A. C., Wall J., Hainfeld J., Steinert P. M. Structure of fibroblastic intermediate filaments: analysis of scanning transmission electron microscopy. Proc Natl Acad Sci U S A. 1982 May;79(10):3101–3105. doi: 10.1073/pnas.79.10.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki E., Crewther W. G., Fraser R. D., MacRae T. P., McKern N. M. X-ray diffraction and infrared studies of an -helical fragment from -keratin. J Mol Biol. 1973 Jan 10;73(2):275–278. doi: 10.1016/0022-2836(73)90329-x. [DOI] [PubMed] [Google Scholar]
- Woods E. F., Gruen L. C. Structural studies on the microfibrillar proteins of wool: characterization of the alpha-helix-rich particle produced by chymotryptic digestion. Aust J Biol Sci. 1981;34(5-6):515–526. doi: 10.1071/bi9810515. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]



