Abstract
Background
Presacral cysts are rare congenital lesions predominantly affecting females. Surgery is often recommended after diagnosis due to the risk of malignant transformation and complications associated with cyst enlargement. Laparoscopic excision is increasingly favored due to its enhanced visualization and precision.
Aim
To assess long-term urinary, sexual function outcomes and quality of life in female patients undergoing laparoscopic resection of presacral cysts.
Methods
We conducted a retrospective review of female patients who underwent laparoscopic resection of presacral cysts between August 2012 and May 2020. Patient demographics, surgical outcomes, and postoperative complications were analyzed. The urinary function was assessed using the International Consultation on Incontinence Questionnaire Female Lower Urinary Tract Symptoms Module (ICIQ-FLUTS), the sexual function was evaluated using the Female Sexual Function Index (FSFI), and quality of life was assessed using the 36-item Short-Form Health Survey (SF-36).
Results
Among the 32 female patients included, 10 experienced postoperative urinary incontinence, predominantly of the mixed type. The risk factors for urinary incontinence included abdominal distension and the proximity of the cyst to the rectum. Notably, urinary incontinence significantly impacted the overall lower urinary tract symptoms and quality of life. Additionally, seven patients reported postoperative sexual dysfunction, with previous abdominal or pelvic surgery and cyst location under S3 identified as risk factors, affecting the mental health aspects of their quality of life.
Conclusion
Laparoscopic cyst resection in females poses risks of urinary and sexual dysfunction, potentially impacting quality of life. Thus, tailored management approaches are crucial.
Keywords: Laparoscopic resection, Presacral cysts, Urinary function impairment, Sexual function impairment, Risk factors, Quality of life
Core tip: We presented a comprehensive examination of laparoscopic resection of presacral cysts, emphasizing the risk factors associated with urinary and sexual dysfunction post-surgery in female patients. Highlighting the rarity of these cysts and consequent gaps in clinical expertise, it underscores the necessity of precise surgical approaches and careful intraoperative technique to minimize complications. It establishes that while laparoscopic methods afford significant visibility and reduce operative trauma, they also pose risks particularly affecting urinary function and sexual health, which profoundly impact patient quality of life. Therefore, the operation should be conducted in medical centers by experienced surgeons.
1. Introduction
Presacral cysts present as cystic masses located between the sacrum and the rectum [1,2]. They are primarily congenital and are more prevalent in females, with a male-to-female incidence ratio of approximately 1:3 [3]. The clinical occurrence of presacral cysts is rare, with an incidence rate of approximately 1 in 40,000 [4], leading to a lack of widespread expertise in their diagnosis and treatment [5,6]. Surgical intervention is typically recommended when diagnosed for several reasons [[7], [8], [9]]. Firstly, there is a risk of malignant transformation, particularly in cystic teratomas, ranging from 5 % to 10 % [[9], [10], [11]]. Secondly, untreated cysts may progressively enlarge, complicating complete excision [12]. Finally, as the cyst enlarges, there is a heightened risk of rupture and secondary infection [13,14].
Presacral cysts often have an insidious onset and slow progression, typically asymptomatic in their early stages [2]. However, as they grow, they may compress nearby organs or tissues, resulting in symptoms such as urinary retention, bladder irritation, or difficulty in defecation due to rectum compression [8,15]. These cysts are located deep within the body, surrounded by complex anatomical structures, such as the coccygeal ligament, levator ani muscle, and coccygeal muscle, which contain loose connective tissue, branches of the sacral plexus, high branches of the sympathetic nerve, and blood vessels [12]. Given the relative complexity of the surrounding anatomy and close adhesions to the rectal wall, surgical procedures require experienced surgeons proficient in rectal and pelvic surgeries to minimize the risk of intraoperative complications, such as rectal injury, as well as the risks of intraoperative omission and residual cyst walls, thereby reducing the incidence of cyst recurrence.
Presacral cysts are closely associated with the presacral fascia, sacrum, and adjacent structures, including the rectum and vagina, often forming dense adhesions [7,15]. During surgical separation, there is a potential risk of damaging the left and right inferior hypogastric nerves descending anterior to the abdominal aorta, as well as the parasympathetic pelvic plexus that innervates the rectum and urinary and genital organs [12], which can impact the bowel, as well as bladder, and sexual functions. Patients undergoing surgery for presacral cysts may experience symptoms such as increased bowel movements, urinary frequency, difficulty in voiding, and impaired sexual function, which improve to varying degrees during the postoperative recovery period. As a result, given the increasing emphasis on quality of life, surgeons need to consider postoperative quality of life and sexual function recovery in these patients.
Laparoscopy offers surgeons several advantages, such as high-definition and magnified operative fields, improved visibility in narrow pelvic spaces, and clearer delineation of anatomical structures, thereby enhancing surgical precision. Since 2012, our center has performed laparoscopic excisions of presacral cysts, completing 74 cases by January 2021 and establishing a prospective clinical database [16]. In our prior study (yet unpublished), we documented the clinical characteristics of this patient cohort, focusing on analyzing factors affecting bowel function. However, to the best of our knowledge, there has been no systematic investigation into long-term complications following presacral cyst surgery. For female patients, urinary dysfunction and impaired sexual function are critical postoperative complications that must not be overlooked. Consequently, we conducted a systematic questionnaire survey on the postoperative urinary function and sexual life of female patients to assess the incidence of related complications following laparoscopic excision of presacral cysts. We aim to provide valuable data and insights for future research in various medical centers.
2. Materials and methods
2.1. Patient characteristics
In this study, we retrospectively reviewed the medical records of female patients who underwent laparoscopic resection of presacral cysts at our hospital between August 2012 and May 2020. The inclusion criteria comprised: (1) female gender; (2) pre-surgery diagnosis of a presacral cyst; (3) laparoscopic cyst resection with or without the transsacral approach; and (4) completion of the follow-up questionnaire. The exclusion criteria encompassed (1) open abdominal or transsacral procedures and (2) surgical pathology reports indicating solid tumors such as lipomas, fibromas, gastrointestinal stromal tumors, and neuroendocrine tumors. We collected patients’ baseline information, clinical presentations, imaging evaluations, surgical outcomes, and pathological diagnoses. Details of the surgical procedures have been described in our prior study [16]. This study was approved by the Ethics Committee of Peking Union Medical College Hospital (approval number: S-K1493), and written informed consent was obtained from all patients.
2.2. Measurements
We utilized the International Consultation on Incontinence Questionnaire Female Lower Urinary Tract Symptoms Module (ICIQ-FLUTS) to assess post-surgery urinary function [17,18]. The ICIQ-FLUTS questionnaire consists of filling scores ranging from 0 to 16, voiding scores ranging from 0 to 12, incontinence scores ranging from 0 to 20, and quality-of-life scores ranging from 0 to 76 [19]. Higher scores on these scales indicate poorer outcomes. Patients were categorized into groups based on self-reported urinary incontinence (UI), with further subgroups for stress UI (SUI), urge UI (UUI), and mixed UI (MUI) [20]. Various patient parameters, including age, body mass index (BMI), American Society of Anesthesiologists (ASA) classification, history of abdominal surgery, clinical manifestations, rectal examination findings, operation duration, blood loss, perioperative complications, and postoperative length of hospital stay, were compared between these groups. The ASA classification reflects the comorbidities in some patients.
For the assessment of sexual function post-surgery [21], we employed the Female Sexual Function Index (FSFI) questionnaire, which evaluates desire, arousal, lubrication, orgasm, satisfaction, and pain during intercourse. Scores on the FSFI range from 0 to 6, with lower scores indicating poorer outcomes. Patients were divided into groups based on self-reported post-surgery pain during intercourse, and clinical characteristics were compared between these groups. Additionally, patients’ overall health status was assessed using the 36-item Short-Form Health Survey (SF-36) questionnaire [22], which includes eight subscales: physical functioning (PF), role-physical (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role-emotional (RE), and mental health (MH). The SF-36 also includes health transition (HT), which evaluates the overall health status in the past year. Scores range from 0 (worst outcome) to 100 (best outcome) for each subscale, with no specific cutoff point to differentiate between good and poor outcomes. Patients self-administered the questionnaires through telephone interviews based on provided instructions. To mitigate recall bias, we cross-checked questionnaire responses with postoperative outpatient medical records to verify the presence or absence of symptoms, since all patients underwent regular postoperative outpatient follow-ups at 1, 6, and 12 months after surgery, during which urinary and sexual symptoms were recorded.
2.3. Statistical analysis
All statistical analyses were performed using SPSS statistical software (version 26.0, for Windows). Variables following a normal distribution were presented as mean ± standard deviation (SD). Variables that did not follow a normal distribution were presented as median and interquartile range (IQR). Quantitative data were analyzed using the t-test and rank-sum test, while enumeration data were assessed using χ2 and Fisher tests and reported as numbers or percentages. Statistical significance was set at P < 0.05.
3. Results
3.1. Risk factors for urinary incontinence
We investigated UI and sexual function impairment in female patients after surgery for presacral cysts. Using the ICIQ-FLUTS, we categorized UI into SUI, UUI, and MUI [20,23], as diagnosed using the ICIQ-FLUTS. Of the 32 female patients who completed follow-up, 10 reported UI post-surgery. All patients did not have related symptoms before surgery, confirmed by their medical records and telephone interviews. Among them, 3 (30 %) had SUI, 1 (10 %) had UUI, and the remaining 6 (60 %) had MUI. Subsequently, we compared the baseline clinical characteristics of the 10 patients with UI and 22 without UI (Table 1). The mean age was 40.1 ± 14.4 years for the UI group and 32.8 ± 11.5 years for the non-UI group, with no significant difference observed. However, patients in the UI group patients tended to be more symptomatic than those in the non-UI group (P = 0.077, odds ratio (OR): 4.083, 95 % confidence interval (CI): 0.818–20.376). Specifically, 3 out of 10 (30.0 %) patients in the UI group reported abdominal distension before surgery, compared to only 1 out of 22 (4.5 %) patients in the non-UI group. Regarding imaging evaluation, there were no significant differences between the two groups in terms of size (major axis), relationship with S3 or the pelvic floor muscle, and presence of polycystic lesions. However, a higher proportion of patients in the UI group (6/10, 60.0 %) had lesions that passed through the opposite edge of the rectum compared to the non-UI group (4/22, 18.2 %; P = 0.018, OR: 6.750, 95 % CI: 1.276–35.701). Both groups exhibited similar surgical outcomes, including ASA classification, operation duration, blood loss, perioperative complications, and postoperative length of stay.
Table 1.
Risk factors for urinary incontinence.
| Urinary incontinence (n = 10) | Non-urinary incontinence (n = 22) | P value | Odds ratio (95 % CI) | |
|---|---|---|---|---|
| Age (y) | 40.1 ± 14.4 | 32.8 ± 11.5 | 0.133 | – |
| BMI (kg/m2) | 24.1 ± 4.8 | 22.3 ± 3.6 | 0.261 | – |
| ≥ 26 kg/m2 | 3 (30.0) | 4 (18.2) | 0.454 | 1.929 (0.341–10.910) |
| Hypertension | 1 (10.0) | 3 (13.6) | 0.773 | 0.704 (0.064–7.742) |
| Diabetes mellitus | 1 (10.0) | 0 (0) | 0.132 | – |
| Previous abdominal and pelvic surgery | 7 (70.0) | 9 (40.9) | 0.127 | 3.370 (0.682–16.650) |
| Symptomatic | 7 (70.0) | 8 (36.4) | 0.077 | 4.083 (0.818–20.376) |
| Bowel habit change | 1 (10.0) | 2 (9.1) | 0.935 | 1.111 (0.089–13.894) |
| Frequent micturition/Dysuria | 0 (0) | 0 (0) | 1.000 | – |
| Abdominal pain/Backache | 3 (30.0) | 5 (22.7) | 0.660 | 1.457 (0.271–7.821) |
| Abdominal distension | 3 (30.0) | 1 (4.5) | 0.044 | 9.000 (0.801–101.155) |
| Anal discomfort | 2 (20.0) | 2 (9.1) | 0.387 | 2.500 (0.299–20.923) |
| Positive digital rectal examination | 6 (60.0) | 18 (85.7) | 0.109 | 0.250 (0.043–1.452) |
| Imaging evaluation | ||||
| Major axis (cm) | 7.5 ± 2.6 | 7.4 ± 2.0 | 0.901 | – |
| 0–5.9 cm | 4 (40.0) | 5 (22.7) | 0.204 | – |
| 6.0–9.9 cm | 3 (30.0) | 14 (63.6) | ||
| 10.0–13.9 cm | 3 (30.0) | 3 (13.6) | ||
| Cross section | 0.018 | 6.750 (1.276–35.701) | ||
| Posterior or lateral to rectum | 4 (40.0) | 18 (81.8) | ||
| Adjacent side of the rectum | 6 (60.0) | 4 (18.2) | ||
| Height | 0.387 | 2.000 (0.412–9.712) | ||
| Above S3 | 5 (50.0) | 12 (66.7) | ||
| Under S3 | 5 (50.0) | 6 (33.3) | ||
| Pelvic floor muscle invasion | 4 (40.0) | 7 (31.8) | 0.652 | 1.429 (0.303–6.737) |
| Polycystic | 6 (60.0) | 11 (50.0) | 0.599 | 1.500 (0.329–6.833) |
| ASA classification | 0.725 | 0.750 (0.150–3.742) | ||
| Class I | 7 (70.0) | 14 (63.6) | ||
| Class II | 3 (30.0) | 8 (36.4) | ||
| Operation duration (min) | 140.1 ± 50.4 | 130.0 ± 62.6 | 0.655 | – |
| Blood loss (mL) | 91.0 ± 148.9 | 39.5 ± 49.1 | 0.150 | – |
| Perioperative complications | 3 (30.0) | 10 (45.5) | 0.409 | 0.514 (0.105–2.526) |
| Postoperative length of stay (d) | 5.5 ± 1.4 | 7.0 ± 4.7 | 0.320 | – |
Data are presented in n (%) for categorical variables and mean ± standard deviation (SD) for continuous variables.
3.2. Influence of urinary incontinence on life quality
The diagnosis of UI was established based on the ICIQ-FLUTS incontinence section. This questionnaire was also employed to evaluate other female lower urinary tract symptoms, including the filling and voiding sections and the overall quality-of-life score. We compared the scores in these sections between the two groups and found significant differences across all sections (Table 2). The mean total score was 13.5 ± 9.3 for the UI group and 3.0 ± 4.3 years for the non-UI group (P = 0.006), indicating that overall lower urinary tract symptoms were more prominent in the UI group, not only in terms of incontinence but also in filling and voiding aspects. The quality of life score reflected the impact of lower urinary tract symptoms on quality of life, with higher scores indicating poorer outcomes. The mean quality of life score was 23.3 ± 33.4 for the UI group and 6.4 ± 10.9 years for the non-UI group (P = 0.038). Additionally, the mean duration of symptoms was significantly longer in the UI group (P = 0.036).
Table 2.
Impact of urinary incontinence on lower urinary tract symptoms and quality of life.
| Urinary function injury (n = 10) | Non-urinary function injury (n = 22) | P value | |
|---|---|---|---|
| ICIQ-FLUTS score | 13.5 ± 9.3 | 3.0 ± 4.3 | 0.006 |
| ICIQ-FLUTS filling score | 4.9 ± 3.7 | 2.2 ± 2.1 | 0.015 |
| ICIQ-FLUTS voiding score | 4.2 ± 4.8 | 0.8 ± 2.4 | 0.011 |
| ICIQ-FLUTS incontinence score | 4.4 ± 3.1 | 0 ± 0 | <0.001 |
| ICIQ-FLUTS quality-of-life score | 23.3 ± 33.4 | 6.4 ± 10.9 | 0.038 |
| Duration of symptoms | 26.7 ± 17.4 (n = 7) | 7.6 ± 19.0 (n = 15) | 0.036 |
Data are presented in mean ± standard deviation (SD).
3.3. Risk factors for sexual function impairment
The impairment of sexual function was another notable postoperative complication evaluated using the FSFI questionnaire. Among the 30 female patients who completed follow-up, 16 reported no sexual activity after surgery; thus, the analysis included results from 14 patients. Similarly, all patients did not have related symptoms before surgery, confirmed by their medical records and telephone interviews. Among these, 7 patients reported experiencing vaginal pain or discomfort during intercourse after surgery, forming the sexual function impairment group. We compared the baseline clinical characteristics between the sexual function impairment and non-sexual function impairment groups (Table 3) and found no significant differences in age, BMI, or comorbidities such as hypertension and diabetes mellitus. However, a higher proportion of patients in the sexual function impairment group (6/7, 85.7 %) had previously undergone abdominal or pelvic surgery, including three cesarean sections, appendectomy, open drainage of pelvic abscess, and sub-coccygeal teratoma resection, compared to only 1 out of 7 (14.3 %) patients in the non-sexual function impairment group. Postoperative symptoms were similar in both groups, and imaging evaluation revealed no significant differences in lesion size, relationship to pelvic muscle, or presence of polycystic lesions. The relationship between the lesion's cross-section and the opposite edge of the rectum showed marginal significance (P = 0.072, OR: 7.500, 95 % CI: 0.759–74.157). Furthermore, in the sexual function impairment group, 5 out of 6 (83.3 %) lesions were located under S3, whereas only 2 out of 7 (28.6 %) lesions in the non-sexual function impairment group were located under S3, with this difference being statistically significant (P = 0.048, OR: 12.500, 95 % CI: 0.839–186.299). Both groups exhibited similar surgical outcomes, including ASA classification, operation duration, blood loss, and perioperative complications. However, the mean postoperative length of stay was slightly longer in the sexual function impairment group (P = 0.088).
Table 3.
Risk factors for sexual function impairment.
| Sexual function injury (n = 7) | Non-sexual function injury (n = 7) | P value | Odds ratio (95 % CI) | |
|---|---|---|---|---|
| Age (y) | 31.4 ± 10.0 | 34.6 ± 9.8 | 0.564 | – |
| BMI (kg/m2) | 21.9 ± 2.7 | 22.7 ± 2.6 | 0.584 | – |
| ≥ 26 kg/m2 | 0 (0) | 1 (12.5) | 0.333 | – |
| Hypertension | 0 (0) | 0 (0) | 1.000 | – |
| Diabetes mellitus | 0 (0) | 0 (0) | 1.000 | – |
| Previous abdominal and pelvic surgery | 6 (85.7) | 1 (14.3) | 0.008 | 0.028 (0.001–0.555) |
| Symptomatic | 4 (57.1) | 3 (42.9) | 0.593 | 1.778 (0.214–14.767) |
| Bowel habit change | 1 (14.3) | 1 (14.3) | 1.000 | 1.000 (0.050–19.963) |
| Frequent micturition/Dysuria | 0 (0) | 0 (0) | 1.000 | – |
| Abdominal pain/Backache | 1 (14.3) | 2 (28.6) | 0.515 | 0.417 (0.029–6.064) |
| Abdominal distension | 0 (0) | 1 (12.5) | 0.333 | – |
| Anal discomfort | 3 (42.9) | 1 (12.5) | 0.185 | 5.250 (0.400–68.946) |
| Positive digital rectal examination | 7 (100) | 6 (75.0) | 0.155 | – |
| Imaging evaluation | ||||
| Major axis (cm) | 6.9 ± 2.8 | 8.2 ± 2.3 | 0.369 | – |
| 0–5.9 cm | 4 (57.1) | 2 (25.0) | ||
| 6.0–9.9 cm | 1 (14.3) | 4 (50.0) | 0.300 | – |
| 10.0–13.9 cm | 2 (28.6) | 2 (25.0) | ||
| Cross section | 0.072 | 7.500 (0.759–74.157) | ||
| Posterior or lateral to rectum | 2 (28.6) | 6 (75.0) | ||
| Adjacent side of the rectum | 5 (71.4) | 2 (25.0) | ||
| Height | 0.048 | 12.500 (0.839–186.299) | ||
| Above S3 | 1/6 (16.7) | 5/7 (71.4) | ||
| Under S3 | 5/6 (83.3) | 2/7 (28.6) | ||
| Pelvic floor muscle invasion | 4 (57.1) | 4 (50.0) | 0.782 | 1.333 (0.173–10.254) |
| Polycystic | 5 (71.4) | 3 (37.5) | 0.189 | 4.167 (0.473–36.736) |
| ASA classification | 0.185 | 5.250 (0.400–68.946) | ||
| Class I | 4 (57.1) | 7 (87.5) | ||
| Class II | 3 (42.9) | 1 (12.5) | ||
| Operation duration (min) | 146.4 ± 72.1 | 129.9 ± 54.7 | 0.637 | – |
| Blood loss (mL) | 49.3 ± 44.0 | 34.3 ± 32.1 | 0.480 | – |
| Perioperative complications | 5 (71.4) | 3 (37.5) | 0.189 | 4.167 (0.473–36.736) |
| Postoperative length of stay (d) | 8.6 ± 5.1 | 4.9 ± 1.6 | 0.088 | – |
Data are presented in n (%) for categorical variables and mean ± standard deviation (SD) for continuous variables.
3.4. Influence of sexual function impairment on life quality
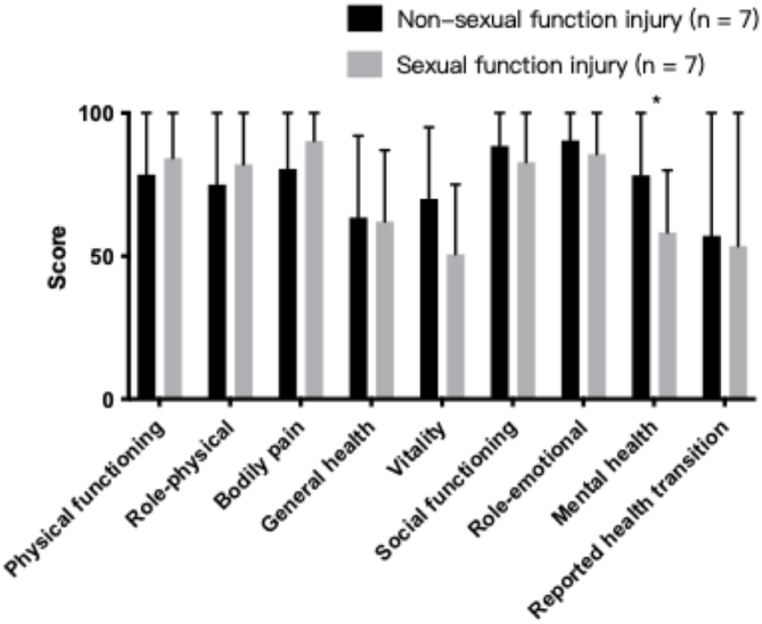
To assess the influence of sexual impairment on quality of life, we compared various aspects of the FSFI questionnaire between the two groups, including desire, arousal, lubrication, orgasm, satisfaction, and pain during intercourse, and found no significant differences between the two groups in all aspects except for the pain score (Table 4). To assess the general health status, we compared the SF-36 questionnaires between the two groups (Fig. 1). Both groups demonstrated similar scores on physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, and reported health transitions. However, the sexual function impairment group exhibited a significantly lower score in mental health (mean difference: 20.00, 95 % CI: 4.82–35.18, P = 0.014).
Table 4.
Impact of sexual function injury on FSFI score.
| Sexual function injury (n = 7) | Non-sexual function injury (n = 7) | P value | |
|---|---|---|---|
| FSFI score | 15.6 ± 6.1 | 20.7 ± 4.4 | 0.105 |
| FSFI desire score | 1.9 ± 0.9 | 2.2 ± 1.3 | 0.580 |
| FSFI arousal score | 2.3 ± 1.0 | 3.5 ± 1.4 | 0.081 |
| FSFI lubrication score | 3.0 ± 1.3 | 3.2 ± 0.9 | 0.781 |
| FSFI orgasm score | 2.8 ± 1.4 | 3.3 ± 0.8 | 0.415 |
| FSFI satisfaction score | 3.0 ± 1.8 | 3.9 ± 1.3 | 0.281 |
| FSFI pain score | 2.6 ± 1.3 | 5.5 ± 0.8 | <0.001 |
Data are presented in mean ± standard deviation (SD).
Fig. 1.
Impact of sexual function impairment on general health. ∗, P < 0.05; ∗∗, P < 0.01.
4. Discussion
Presacral cysts are clinically rare and often misdiagnosed, leading to improper management [6]. Generally, surgical resection is the recommended approach for definitive treatment of this condition. However, comprehensive investigations into long-term surgical complications are lacking due to their rarity. Given the proximity of presacral cysts to adjacent organs, vessels, and nerves, postoperative complications related to defecation, micturition function, and sexual dysfunction are of utmost importance. Indeed, many other pelvic surgeries have similar postoperative complications including urinary and sexual dysfunctions, such as rectal cancer surgery [24] and many gynecological surgeries [25]. Besides direct damage during surgery, inflammation following surgery could also cause nerve damage and thus inducing urinary and sexual dysfunctions [24]. However, due to the rarity of presacral cysts, to our knowledge, this is the first study to systematically summarize the characteristics of these complications and their associated risk factors following laparoscopic resection of presacral cysts. Previous studies have primarily focused on the risk of voiding difficulties following surgery [12], but our findings highlighted that in female patients, micturition dysfunction can manifest as difficulties in filling, voiding, and urinary incontinence, an aspect prominent but often overlooked previously. This discrepancy may stem from our exclusive inclusion of female patients in the study.
UI in women is a prevalent and multifaceted condition characterized by the involuntary leakage of urine [23]. The types of UI include SUI, UUI, and MUI. SUI occurs when pressure or sudden muscle contractions in the bladder lead to unexpected urine leakage, typically triggered by activities such as coughing, sneezing, laughter, or exercising. UUI, also known as overactive bladder (OAB), involves a sudden and strong urge to urinate due to bladder contractions, resulting in involuntary urine leakage. MUI is a combination of both SUI and UUI in which individuals experience symptoms of both stress and urge incontinence. Our results revealed that 6 out of 10 (60 %) patients with UI had MUI, suggesting variability in the underlying pathophysiology. Potential mechanisms contributing to postoperative UI include inadvertent damage to the pelvic splanchnic nerves crucial for bladder control during laparoscopic procedures [26,27], alterations in pelvic floor anatomy affecting urethral and bladder neck support, leading to SUI, and inflammatory responses after surgery impacting bladder and urethral tissues, potentially exacerbating preexisting UI or causing new-onset UI.
In our analysis of risk factors for UI, we found that abdominal distension and the cross-section of the lesion significantly impacted the emergence of UI. Abdominal distension may be attributed to larger cyst sizes and their proximity to adjacent organs, while the cross-section of the lesion has been associated with a higher likelihood of defecation dysfunction, suggesting its importance as an indicator of surgical complexity. A larger cross-section could pose challenges in fully separating and resecting the lesion without interfering with adjacent nerves and vessels. While previous research has identified lesion size [16] and height (above or below S3) [28] as risk factors for preoperative complications, our findings did not support their significance in predicting long-term complications. Moreover, we observed that patients with postoperative UI were more susceptible to other lower urinary tract symptoms, such as filling and voiding problems, which persisted over time. UI has been widely documented to negatively impact the quality of life across various domains, including physical, mental, sexual, and social health [29,30]. Research has also shown that the type of UI also played a role in its impact, with reports suggesting that MUI and UUI had more detrimental effects than SUI. This could be attributed to the fact that patients with SUI usually tend to avoid triggering situations to alleviate symptoms. However, we did not investigate this relationship because of the predominance of MUI among our patients; thus, further investigation into the relationship between UI type and its impact is warranted.
Pain during intercourse emerged as a common surgical complication, with half of the patients reporting vaginal pain or discomfort during sex post-surgery. While various medical causes contribute to painful intercourse, including endometriosis, pelvic infection, and atrophic vaginitis, postoperative pain is more likely attributed to anatomical changes and damage to pelvic visceral nerves [31]. Our results highlighted a previous history of abdominal and pelvic surgery as a significant risk factor, probably due to resulting adhesions in the operative area. The cross-section also had marginal significance, possibly due to its contribution to operative difficulties. Another notable risk factor was the relationship between the lesion and S3. We observed that patients with presacral cysts under S3 were more susceptible to sexual dysfunction after surgery, possibly due to their proximity to the reproductive system.
The impact of sexual dysfunction on quality of life was particularly pronounced in aspects related to mental health, while it did not significantly alter aspects concerning physical functioning, general health, or bodily pain. Although we defined sexual dysfunction as vaginal pain or discomfort during intercourse postoperatively, the bodily pain subscale primarily focused on how pain interfered with daily work. This might explain why there was no significant difference in bodily pain scores. Mental health scores, derived from questions about feelings of nervousness, sadness, calmness, and overall happiness, provide a broad measure of psychological well-being. Therefore, it is difficult to definitively conclude that mental health is directly impacted by sexual dysfunction based on our data. Larger studies are necessary to further explore this relationship. Nevertheless, surgeons should remain aware of these potential complications and their possible effects on patients' overall quality of life.
To minimize the incidence of postoperative complications, several anatomical considerations during the resection of presacral lesions are crucial, such as the protection of the vagina in female patients, avoidance of rectal injury, and preservation of the inferior hypogastric plexus and sacral veins [12]. Hypogastric nerves could serve as an important anatomical landmark of pelvic autonomic nerves during surgery, but surgeons should be familiar with anatomical cartography of the hypogastric nerves [32]. During dissection of lesions, surgeons should meticulously follow the smooth texture of the true cyst wall, progressing anteriorly, laterally, and posteriorly from the top. Special attention is needed at the termination of the rectal mesentery, where the intestinal wall is thinnest, and adhesions are most significant. Moreover, understanding the pelvic floor muscles' fascial thickening and strategically decompressing large cysts near their bases can help reduce surgical complexity. This comprehensive approach aims to minimize the risk of recurrence, reduce operative difficulty, and ensure patient safety and recovery. Besides, selection of surgical approaches could benefit avoiding surgical injuries. Transperineal approach, for example, could avoid autonomic nerve dissection since it does not require accessing the abdominal cavity [12,33]. The indications for transperineal approach have not been fully sdandardized. Previously, it is recommended for patients with cysts below S3 level [12,34], but further research showed that complete resection of presacral cyst could be reached even if the lesion reached S1 level [35]. However, transperineal approach also has some disadvantages, such as higher infection rate from the close relationship to the anus [36]. Robotic approach is another promising approaching since it offers better visualization and potential higher chance of preservation of autonomic nerves [37]. However, related studies were scarce, and more studies should be conducted to compare the overall efficiencies among different surgical approaches.
Our study was the first to specifically investigate long-term urinary and sexual dysfunction following laparoscopic resection of presacral lesions, identifying their risk factors and assessing their impact on quality of life. While transperineal approaches have been suggested to protect the inferior rectal nerve and deep branches of the perineum nerves, preserving defecation, urination, and sexual function, further patient data and randomized control studies are needed to validate this approach, which currently applies to only a limited number of patients.
Despite its strength, this study has some limitations. Firstly, the sample size was relatively small due to the rarity of the disease. Although our cohort was among the largest reported, the sample size, especially regarding sexual function, remained small during follow-up. Secondly, being a single-center study, the results should be validated using different standardized tests to enhance their generalizability.
5. Conclusion
Laparoscopic resection of presacral cysts in female patients poses risks of urinary and sexual dysfunction, with distinct risk factors identified for each complication. These complications significantly impact the quality of life, particularly in terms of mental health. Consequently, surgeons need to consider these risks and tailor management strategies to optimize patient outcomes. Moreover, further studies with larger cohorts are warranted to validate these findings and enhance their generalizability.
Institutional review board
This study was approved by the Ethics Committee of Peking Union Medical College Hospital (approval number: S-K1493).
Informed consent
The study used anonymized clinical data obtained after each patient provided written informed consent.
Data sharing statement
No additional data are available.
Supported by
National High Level Hospital Clinical Research Funding (2022-PUMCH-B-003), the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2021-1-I2M-015) and Peking Union Medical College Hospital Undergraduate Educational Reform Project (No. 2020zlgc0116, No.2023kcsz004).
CRediT authorship contribution statement
Chen Lin: Writing – review & editing, Writing – original draft, Data curation, Conceptualization. Zi-Yan Wang: Writing – review & editing, Writing – original draft, Data curation. Pei-Pei Wang: Data curation, Conceptualization. Kai-Wen Xu: Data curation, Conceptualization. Jiao-Lin Zhou: Conceptualization. Hui-Zhong Qiu: Data curation, Conceptualization. Bin Wu: Writing – review & editing, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We sincerely thank the patients for their cooperation and participation in this study. We also thank the surgeons, physicians, nurses, technical staff, and hospital administrators for their invaluable contribution to this study. Additionally, we express our sincere gratitude to Dr. Wu for his advice and support. The authors declare no conflict of interest.
References
- 1.Boscà A., Pous S., Artés M.J., Gómez F., Granero Castro P., García‐Granero E. Tumours of the retrorectal space: management and outcome of a heterogeneous group of diseases. Colorectal Dis. 2012;14(11):1418–1423. doi: 10.1111/j.1463-1318.2012.03016.x. [DOI] [PubMed] [Google Scholar]
- 2.Barraqué M., Filippello A., Brek A., Baccot S., Porcheron J., Barabino G. Surgical management of retro-rectal tumors in the adult. J. Vis. Surg. 2019;156(3):229–237. doi: 10.1016/j.jviscsurg.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Hjermstad B.M., Helwig E.B. Tailgut cysts: report of 53 cases. Am. J. Clin. Pathol. 1988;89(2):139–147. doi: 10.1093/ajcp/89.2.139. [DOI] [PubMed] [Google Scholar]
- 4.Jao S.W., Beart R.W., Spencer R.J., Reiman H.M., Ilstrup D.M. Retrorectal tumors: Mayo clinic experience, 1960-1979. Dis. Colon Rectum. 1985;28(9):644–652. doi: 10.1007/BF02553440. [DOI] [PubMed] [Google Scholar]
- 5.Piura B., Rabinovich A., Sinelnikov I., Delgado B. Tailgut cyst initially misdiagnosed as ovarian tumor. Arch. Gynecol. Obstet. 2005;272(4):301–303. doi: 10.1007/s00404-005-0012-3. [DOI] [PubMed] [Google Scholar]
- 6.Singer M.A., Cintron J.R., Martz J.E., Schoetz D.J., Abcarian H. Retrorectal cyst: a rare tumor frequently misdiagnosed. J. Am. Coll. Surg. 2003;196(6):880–886. doi: 10.1016/S1072-7515(03)00133-9. [DOI] [PubMed] [Google Scholar]
- 7.Chéreau N., Lefevre J.H., Meurette G., et al. Surgical resection of retrorectal tumours in adults: long‐term results in 47 patients. Colorectal Dis. 2013;15(8) doi: 10.1111/codi.12255. [DOI] [PubMed] [Google Scholar]
- 8.Glasgow S., Dietz D. Retrorectal tumors. Clin. Colon Rectal Surg. 2006;19(2):61–68. doi: 10.1055/s-2006-942346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glasgow S.C., Birnbaum E.H., Lowney J.K., et al. Retrorectal tumors: a diagnostic and therapeutic challenge. Dis. Colon Rectum. 2005;48(8):1581–1587. doi: 10.1007/s10350-005-0048-2. [DOI] [PubMed] [Google Scholar]
- 10.Hobson K.G., Ghaemmaghami V., Roe J.P., Goodnight J.E., Khatri V.P. Tumors of the retrorectal space. Dis. Colon Rectum. 2005;48(10):1964–1974. doi: 10.1007/s10350-005-0122-9. [DOI] [PubMed] [Google Scholar]
- 11.Wohlmuth C., Bergh E., Bell C., et al. Clinical monitoring of sacrococcygeal teratoma. Fetal Diagn. Ther. 2019;46(5):333–340. doi: 10.1159/000496841. [DOI] [PubMed] [Google Scholar]
- 12.Wang G., Miao C. Chinese expert consensus on standardized treatment for presacral cysts. Gastroenterol Rep. 2022;11 doi: 10.1093/gastro/goac079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abel M.E., Nelson R., Prasad L.M., Pearl R.K., Orsay C.P., Abcarian H. Parasacrococcygeal approach for the resection of retrorectal developmental cysts. Dis. Colon Rectum. 1985;28(10):855–858. doi: 10.1007/BF02555492. [DOI] [PubMed] [Google Scholar]
- 14.Gao X.H., Zhang W., Fu C.G., Liu L.J., Yu E.D., Meng R.G. Local recurrence after intended curative excision of presacral lesions: causes and preventions. World J. Surg. 2011;35(9):2134–2142. doi: 10.1007/s00268-011-1155-y. [DOI] [PubMed] [Google Scholar]
- 15.Teng L., Lin Jin. Surgical management of retrorectal tumors: a retrospective study of a 9-year experience in a single institution. OncoTargets Ther. November 2011;203 doi: 10.2147/OTT.S25271. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang P.P., Lin C., Zhou J.L., Xu K.W., Qiu H.Z., Wu B. Risk factors for perioperative complications in laparoscopic surgeries of retrorectal cystic lesions. World J. Gastrointest. Surg. 2021;13(12):1685–1695. doi: 10.4240/wjgs.v13.i12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Przydacz M., Dudek P., Chlosta P. Polish versions of the ICIQ-FLUTS and the ICIQ-FLUTS LF: translation, adaptation, and validation of female-specific instruments for evaluation of lower urinary tract symptoms. Int Urogynecology J. 2021;32(12):3259–3265. doi: 10.1007/s00192-021-04793-z. [DOI] [PubMed] [Google Scholar]
- 18.Huang L., Zhang S.W., Wu S.L., Ma L., Hong D.X. The Chinese version of ICIQ: a useful tool in clinical practice and research on urinary incontinence. Neurourol. Urodyn. 2008;27(6):522–524. doi: 10.1002/nau.20546. [DOI] [PubMed] [Google Scholar]
- 19.Abdel-Fattah M., Cooper D., Davidson T., et al. Single-Incision mini-slings for stress urinary incontinence in women. N. Engl. J. Med. 2022;386(13):1230–1243. doi: 10.1056/NEJMoa2111815. [DOI] [PubMed] [Google Scholar]
- 20.Samuelsson E., Victor A., Tibblin G. A population study of urinary incontinence and nocturia among women aged 20‐59 years. Acta Obstet. Gynecol. Scand. 1997;76(1):74–80. doi: 10.3109/00016349709047789. [DOI] [PubMed] [Google Scholar]
- 21.Rosen C. Brown J., Heiman S. Leib R. The female sexual function index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J. Sex Marital Ther. 2000;26(2):191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 22.Ware J.E., Sherbourne C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 23.Li Z., Xu T., Zhang L., Zhu L. Prevalence, potential risk factors, and symptomatic bother of lower urinary tract symptoms during and after pregnancy. LUTS Low Urin Tract Symptoms. 2019;11(4):217–223. doi: 10.1111/luts.12274. [DOI] [PubMed] [Google Scholar]
- 24.Lange M.M., Van De Velde C.J.H. Urinary and sexual dysfunction after rectal cancer treatment. Nat. Rev. Urol. 2011;8(1):51–57. doi: 10.1038/nrurol.2010.206. [DOI] [PubMed] [Google Scholar]
- 25.Ianieri M.M., Raimondo D., Rosati A., et al. Impact of nerve‐sparing posterolateral parametrial excision for deep infiltrating endometriosis on postoperative bowel, urinary, and sexual function. Int. J. Gynecol. Obstet. 2022;159(1):152–159. doi: 10.1002/ijgo.14089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glazener C., Herbison G., MacArthur C., et al. New postnatal urinary incontinence: obstetric and other risk factors in primiparae. BJOG An Int. J. Obstet. Gynaecol. 2006;113(2):208–217. doi: 10.1111/j.1471-0528.2005.00840.x. [DOI] [PubMed] [Google Scholar]
- 27.Thomason A.D., Miller J.M., DeLancey J.O. Urinary incontinence symptoms during and after pregnancy in continent and incontinent primiparas. Int Urogynecology J. 2006;18(2):147–151. doi: 10.1007/s00192-006-0124-8. [DOI] [PubMed] [Google Scholar]
- 28.Baek S.K., Hwang G.S., Vinci A., et al. Retrorectal tumors: a comprehensive literature review. World J. Surg. 2016;40(8):2001–2015. doi: 10.1007/s00268-016-3501-6. [DOI] [PubMed] [Google Scholar]
- 29.Rothbarth J., Bemelman W.A., Meijerink W.J.H.J., et al. What is the impact of fecal incontinence on quality of life? Dis. Colon Rectum. 2001;44(1):67–71. doi: 10.1007/BF02234823. [DOI] [PubMed] [Google Scholar]
- 30.Saboia D.M., Firmiano M.L.V., Bezerra K.D.C., Vasconcelos Neto J.A., Oriá M.O.B., Vasconcelos C.T.M. Impacto dos tipos de incontinência urinária na qualidade de vida de mulheres. Rev. Esc. Enferm. USP. 2017;51(0) doi: 10.1590/s1980-220x2016032603266. [DOI] [PubMed] [Google Scholar]
- 31.Herbenick D., Schick V., Sanders S.A., Reece M., Fortenberry J.D. Pain experienced during vaginal and anal intercourse with other-sex partners: findings from a nationally representative probability study in the United States. J. Sex. Med. 2015;12(4):1040–1051. doi: 10.1111/jsm.12841. [DOI] [PubMed] [Google Scholar]
- 32.Seracchioli R., Mabrouk M., Mastronardi M., et al. Anatomic cartography of the hypogastric nerves and surgical insights for autonomic preservation during radical pelvic procedures. J. Minim. Invasive Gynecol. 2019;26(7):1340–1345. doi: 10.1016/j.jmig.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Aranda-Narváez J.M. Posterior approach (Kraske procedure) for surgical treatment of presacral tumors. World J. Gastrointest. Surg. 2012;4(5):126. doi: 10.4240/wjgs.v4.i5.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oguz A., Böyük A., Turkoglu A., et al. Retrorectal tumors in adults: a 10-year retrospective study. Int. Surg. 2015;100(7–8):1177–1184. doi: 10.9738/INTSURG-D-15-00068.1. [DOI] [PubMed] [Google Scholar]
- 35.Hopper L., Eglinton T.W., Wakeman C., Dobbs B.R., Dixon L., Frizelle F.A. Progress in the management of retrorectal tumours. Colorectal Dis. 2016;18(4):410–417. doi: 10.1111/codi.13117. [DOI] [PubMed] [Google Scholar]
- 36.Zhao X., Zhou S., Liu N., Li P., Chen L. Is there another posterior approach for presacral tumors besides the Kraske procedure? — a study on the feasibility and safety of surgical resection of primary presacral tumors via transsacrococcygeal transverse incision. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.892027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim H.S., Oh B., Chung S.S., Lee R., Noh G.T. Trans‐abdominal single‐incision robotic surgery with the da Vinci SP® surgical system for 8 cases of retrorectal tumour. Int J Med Robot. 2024;20(1) doi: 10.1002/rcs.2599. [DOI] [PubMed] [Google Scholar]