Abstract
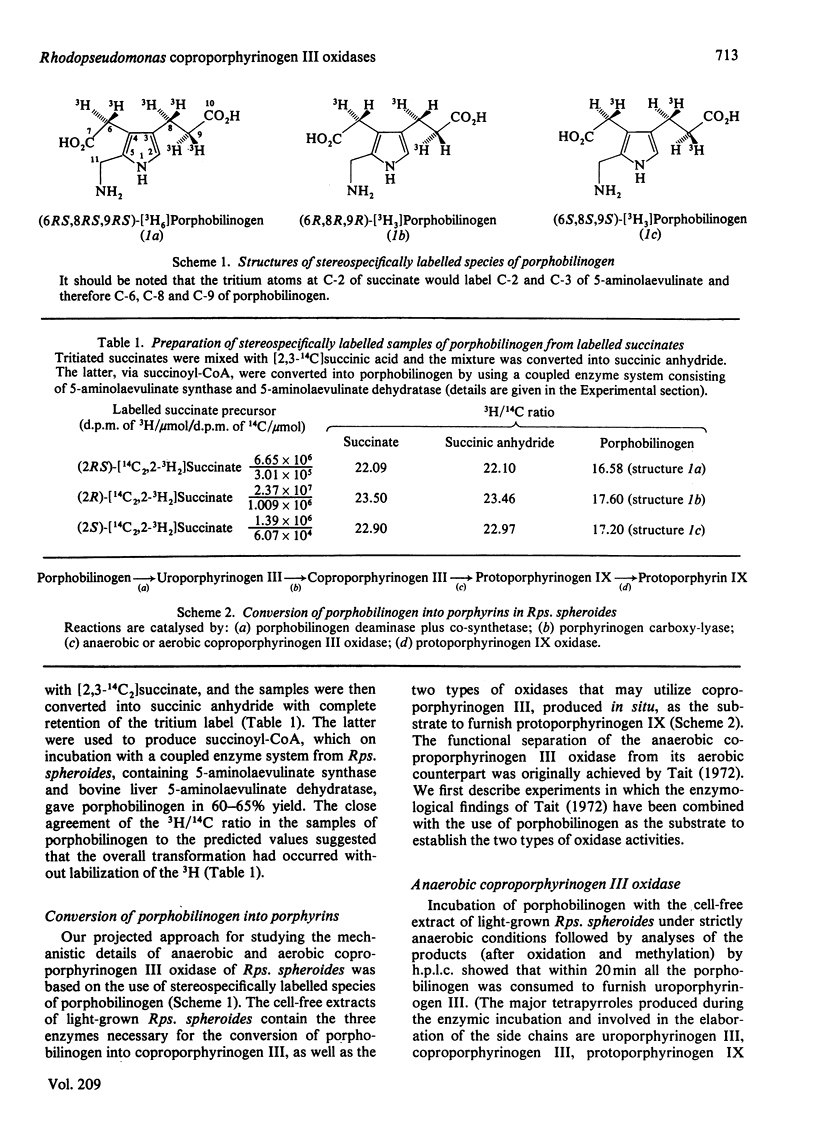
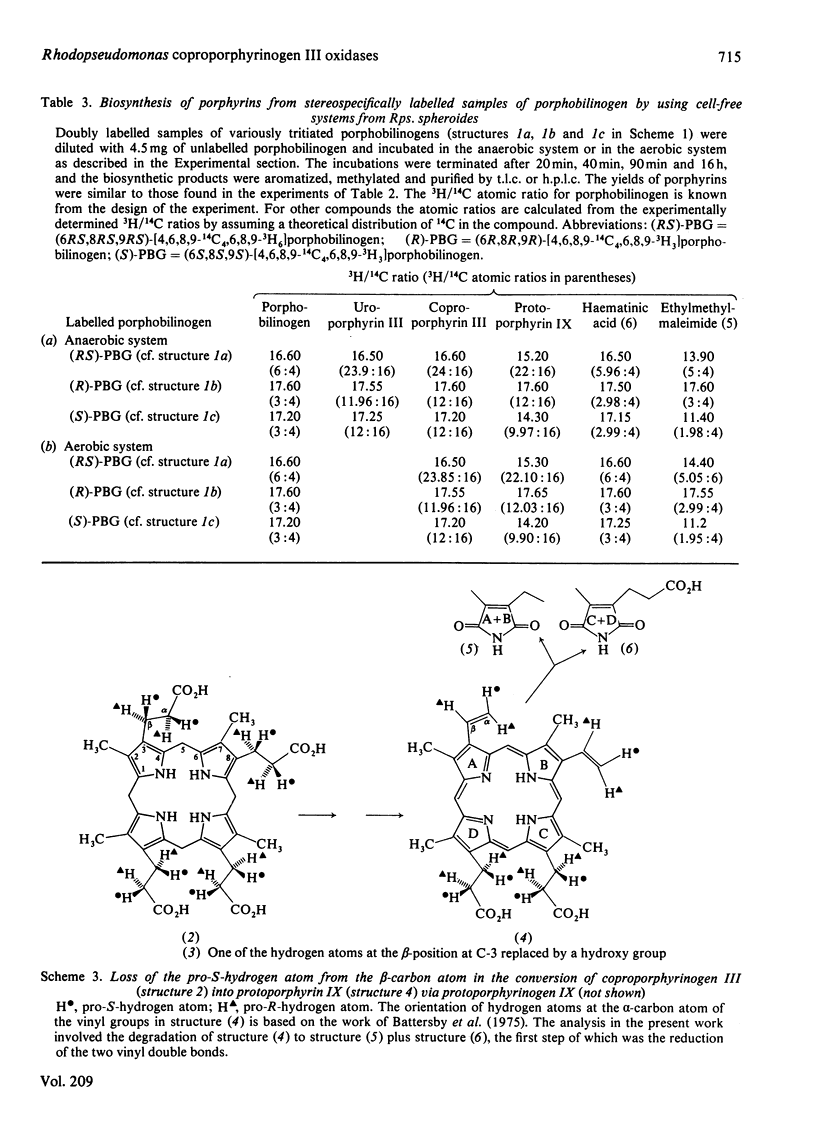
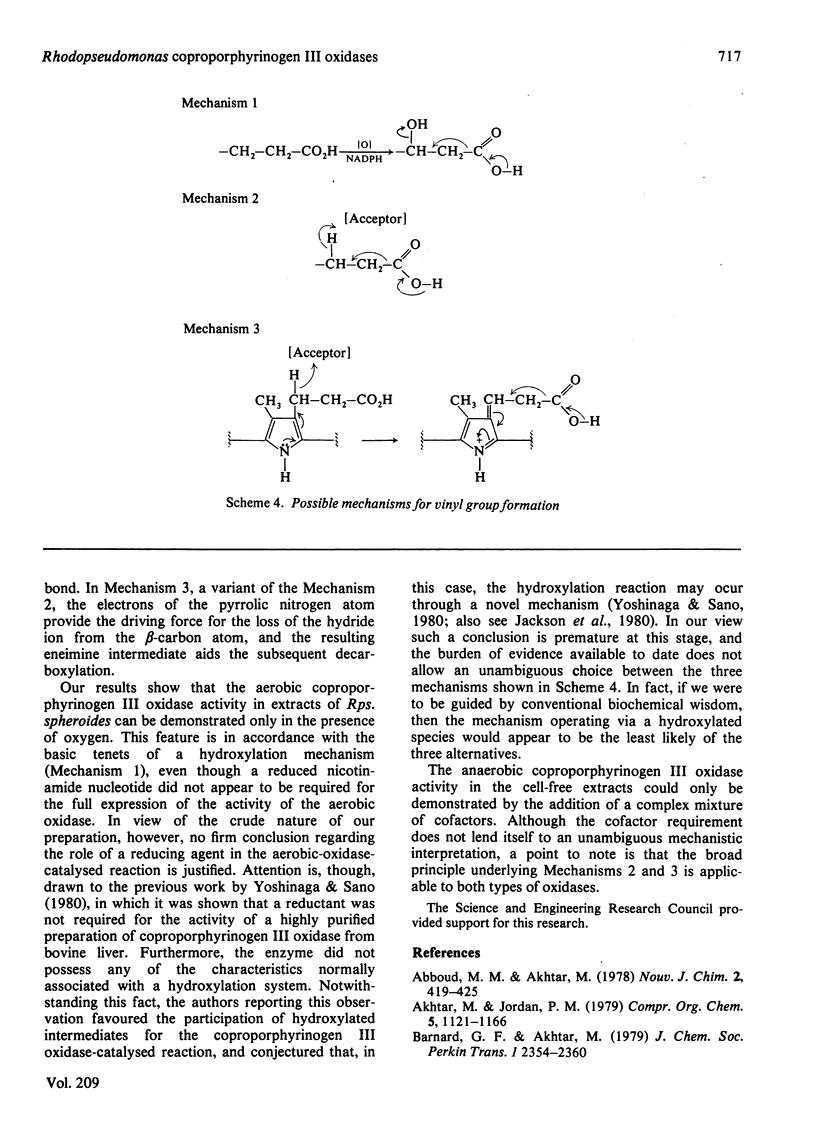
An improved method for the preparation of various species of porphobilinogen stereospecifically labelled with 3H in the side chains (at C-6, C-7 and C-8) is described. These labelled samples were used to study the mechanism and stereochemistry of anaerobic as well as aerobic coproporphyrinogen III oxidase of light-grown Rhodopseudomonas spheroides. It was shown that both the oxidases catalyse the conversion of the propionate side chains of coproporphyrinogen III into the vinyl groups of protoporphyrinogen IX, (formula; see text) with the labilization of the pro-S-hydrogen atom at the beta-position. These results are similar to those previously recorded for such conversions in animal and plant systems. In the light of the cumulative information available to date, mechanisms for the conversion, (formula; see text) are discussed and doubt is cast on the participation of hydroxylated intermediates in the process.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnard G. F., Akhtar M. Stereochemical and mechanistic studies on the decarboxylation of uroporphyrinogen III in haem biosynthesis. J Chem Soc Perkin 1. 1979;10:2354–2360. doi: 10.1039/p19790002354. [DOI] [PubMed] [Google Scholar]
- Battersby A. R. The discovery of nature's biosynthetic pathways. Experientia. 1978 Jan 15;34(1):1–13. doi: 10.1007/BF01921870. [DOI] [PubMed] [Google Scholar]
- Cavaleiro J. A., Kenner G. W., Smith K. M. Pyrroles and related compounds. XXXII. Biosynthesis of protoporphyrin-IX from coproporphyrinogen-3. J Chem Soc Perkin 1. 1974;10(0):1188–1194. doi: 10.1039/p19740001188. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Ehteshamuddin A. F. Anaerobic formation of protoporphyrin IX from coproporphyrinogen III by bacterial preparations. Biochem J. 1968 Apr;107(3):446–447. doi: 10.1042/bj1070446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASCELLES J. The synthesis of porphyrins and bacteriochlorophyll by cell suspensions of Rhodopseudomonas spheroides. Biochem J. 1956 Jan;62(1):78–93. doi: 10.1042/bj0620078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIENHARD G. E., ROSE I. A. THE STEREOCHEMISTRY OF DECARBOXYLATION OF ISOCITRATE BY ISOCITRIC ACID DEHYDROGENASE. Biochemistry. 1964 Feb;3:185–190. doi: 10.1021/bi00890a008. [DOI] [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- ROSE Z. B. Studies on the mechanism of action of isocitric dehydrogenase. J Biol Chem. 1960 Apr;235:928–933. [PubMed] [Google Scholar]
- Tait G. H. Coproporphyrinogenase activities in extracts of Rhodopseudomonas spheroides and Chromatium strain D. Biochem J. 1972 Aug;128(5):1159–1169. doi: 10.1042/bj1281159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga T., Sano S. Coproporphyrinogen oxidase. II. Reaction mechanism and role of tyrosine residues on the activity. J Biol Chem. 1980 May 25;255(10):4727–4731. [PubMed] [Google Scholar]
- Zaman Z., Akhtar M. Mechanism and stereochemistry of vinyl-group formation in haem biosynthesis. Eur J Biochem. 1976 Jan 2;61(1):215–223. doi: 10.1111/j.1432-1033.1976.tb10014.x. [DOI] [PubMed] [Google Scholar]
- Zaman Z., Jordan P. M., Akhtar M. Mechanism and stereochemistry of the 5-aminolaevulinate synthetase reaction. Biochem J. 1973 Oct;135(2):257–263. doi: 10.1042/bj1350257. [DOI] [PMC free article] [PubMed] [Google Scholar]


