Abstract
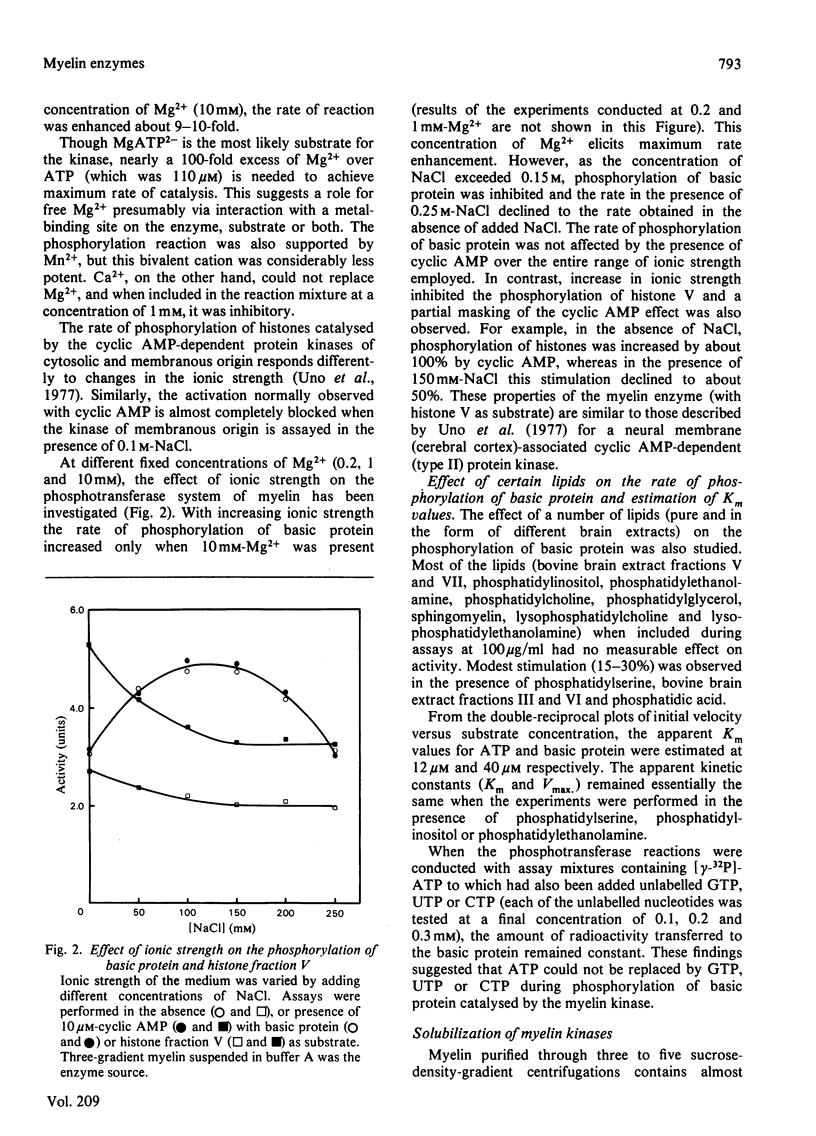
The phosphotransferase system of human central-nervous-system myelin was investigated. Evidence obtained indicated the presence of at least two different phosphotransferase systems (cyclic nucleotide-dependent and -independent) in myelin, which were found to be firmly associated with the membrane. The cyclic AMP-dependent kinase of myelin and white-matter cytosol preferentially phosphorylated certain histone fractions and displayed only modest activity with basic protein as substrate. On the other hand, the cyclic nucleotide-independent system showed specificity toward basic protein. Its activity was not only dependent on Mg2+ but it was greatly enhanced by this bivalent cation. Whereas the cyclic nucleotide-dependent kinase could be extracted with buffers containing Triton X-100, the bivalent cation-regulated kinase resisted solubilization from myelin under these conditions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal H. C., O'Connell K., Randle C. L., Agrawal D. Phosphorylation in vivo of four basic proteins of rat brain myelin. Biochem J. 1982 Jan 1;201(1):39–47. doi: 10.1042/bj2010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carnegie P. R., Dunkley P. R., Kemp B. E., Murray A. W. Phosphorylation of selected serine and threonine residues in myelin basic protein by endogenous and exogenous protein kinases. Nature. 1974 May 10;249(453):147–150. doi: 10.1038/249147a0. [DOI] [PubMed] [Google Scholar]
- Carnegie P. R., Kemp B. E., Dunkley P. R., Murray A. W. Phosphorylation of myelin basic protein by an adenosine 3':5'-cyclic monophosphate-dependent protein kinase. Biochem J. 1973 Nov;135(3):569–572. doi: 10.1042/bj1350569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deibler G. E., Martenson R. E., Kies M. W. Large scale preparation of myelin basic protein from central nervous tissue of several mammalian species. Prep Biochem. 1972;2(2):139–165. doi: 10.1080/00327487208061467. [DOI] [PubMed] [Google Scholar]
- Everly J. L., Quarles R. H., Brady R. O. Proteins and glycoproteins in myelin purified from the developing bovine and human central nervous systems. J Neurochem. 1977 Jan;28(1):95–101. doi: 10.1111/j.1471-4159.1977.tb07713.x. [DOI] [PubMed] [Google Scholar]
- Fricke U. Tritosol: a new scintillation cocktail based on Triton X-100. Anal Biochem. 1975 Feb;63(2):555–558. doi: 10.1016/0003-2697(75)90379-6. [DOI] [PubMed] [Google Scholar]
- Huang K. P., Robinson J. C. A rapid and sensitive assay method for protein kinase. Anal Biochem. 1976 May 7;72:593–599. doi: 10.1016/0003-2697(76)90571-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maeno H., Johnson E. M., Greengard P. Subcellular distribution of adenosine 3',5'-monophosphate-dependent protein kinase in rat brain. J Biol Chem. 1971 Jan 10;246(1):134–142. [PubMed] [Google Scholar]
- Miyamoto E., Kakiuchi S. In vitro and in vivo phosphorylation of myelin basic protein by exogenous and endogenous adenosine 3':5'-monophosphate-dependent protein kinases in brain. J Biol Chem. 1974 May 10;249(9):2769–2777. [PubMed] [Google Scholar]
- Miyamoto E., Kuo J. F., Greengard P. Adenosine 3',5'-monophosphate-dependent protein kinase from brain. Science. 1968 Jul 4;165(3888):63–65. [PubMed] [Google Scholar]
- Miyamoto E., Miyazaki K., Hirose R., Kashiba A. Multiple forms of protein kinases in myelin and microsomal fractions of bovine brain. J Neurochem. 1978 Jul;31(1):269–275. doi: 10.1111/j.1471-4159.1978.tb12459.x. [DOI] [PubMed] [Google Scholar]
- Miyamoto E. Protein kinases in myelin of rat brain: solubilization and characterization. J Neurochem. 1975 Mar;24(3):503–512. doi: 10.1111/j.1471-4159.1975.tb07668.x. [DOI] [PubMed] [Google Scholar]
- Nishiyama K., Katakami H., Yamamura H., Takai Y., Shimomura R. Functional specificity of guanosine 3':5'-monophosphate-dependent and adenosine 3':5'-monophosphate-dependent protein kinases from silkworm. J Biol Chem. 1975 Feb 25;250(4):1297–1300. [PubMed] [Google Scholar]
- Palmer F. B., Dawson R. M. Complex-formation between triphosphoinositide and experimental allergic encephalitogenic protein. Biochem J. 1969 Mar;111(5):637–646. [PMC free article] [PubMed] [Google Scholar]
- Petrali E. H., Thiessen B. J., Sulakhe P. V. Characteristics of magnesium-dependent, Ca2+ -stimulated endogenous protein kinase-catalyzed phosphorylation of basic proteins in myelin isolated from rat brain stem white matter. Arch Biochem Biophys. 1980 Dec;205(2):520–535. doi: 10.1016/0003-9861(80)90135-6. [DOI] [PubMed] [Google Scholar]
- Petrali E. H., Thiessen B. J., Sulakhe P. V. Magnesium ion-dependent, calcium ion stimulated, endogenous protein kinase-catalyzed phosphorylation of basic proteins in myelin fraction of rat brain white matter. Int J Biochem. 1980;11(1):21–36. doi: 10.1016/0020-711x(80)90276-1. [DOI] [PubMed] [Google Scholar]
- Steck A. J., Appel S. H. Phosphorylation of myelin basic protein. J Biol Chem. 1974 Sep 10;249(17):5416–5420. [PubMed] [Google Scholar]
- Sulakhe P. V., Petrali E. H., Davis E. R., Thiessen B. J. Calcium ion stimulated endogenous protein kinase catalyzed phosphorylation of basic proteins in myelin subfractions and myelin-like membrane fraction from rat brain. Biochemistry. 1980 Nov 11;19(23):5363–5371. doi: 10.1021/bi00564a034. [DOI] [PubMed] [Google Scholar]
- Traugh J. A., Ashby C. D., Walsh D. A. Criteria for the classification of protein kinases. Methods Enzymol. 1974;38:290–299. doi: 10.1016/0076-6879(74)38045-7. [DOI] [PubMed] [Google Scholar]
- Uno I., Ueda T., Greengard P. Adenosine 3':5'-monophosphate-regulated phosphoprotein system of neuronal membranes. II. Solubilization, purification, and some properties of an endogenous adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1977 Jul 25;252(14):5164–5174. [PubMed] [Google Scholar]
- Uyemura K., Tobari C., Hirano S., Tsukada Y. Comparative studies on the myelin proteins of bovine peripheral nerve and spinal cord. J Neurochem. 1972 Nov;19(11):2607–2614. doi: 10.1111/j.1471-4159.1972.tb01319.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weller M., Rodnight R. Stimulation by cyclic AMP of intrinsic protein kinase activity in ox brain membrane preparations. Nature. 1970 Jan 10;225(5228):187–188. doi: 10.1038/225187a0. [DOI] [PubMed] [Google Scholar]
- Wu N. C., Martinez J. J., Ahmad F. Phosphoprotein phosphatase of human central nervous system myelin: purification to apparent homogeneity of a low Mr phosphatase and characterization of the high Mr phosphatase. FEBS Lett. 1980 Jul 28;116(2):157–160. doi: 10.1016/0014-5793(80)80632-6. [DOI] [PubMed] [Google Scholar]
- Yourist J. E., Ahmad F., Brady A. H. Solubilization and partial characterization of a phosphoprotein phosphatase from human myelin. Biochim Biophys Acta. 1978 Feb 10;522(2):452–464. doi: 10.1016/0005-2744(78)90078-5. [DOI] [PubMed] [Google Scholar]
- Zetusky W. J., Calabrese V. P., Zetusky A. L., Anderson M. G., Cullen M., DeVries G. H. Isolation and partial characterization of human CNS axolemma-enriched fractions. J Neurochem. 1979 Mar;32(3):1103–1109. doi: 10.1111/j.1471-4159.1979.tb04601.x. [DOI] [PubMed] [Google Scholar]


