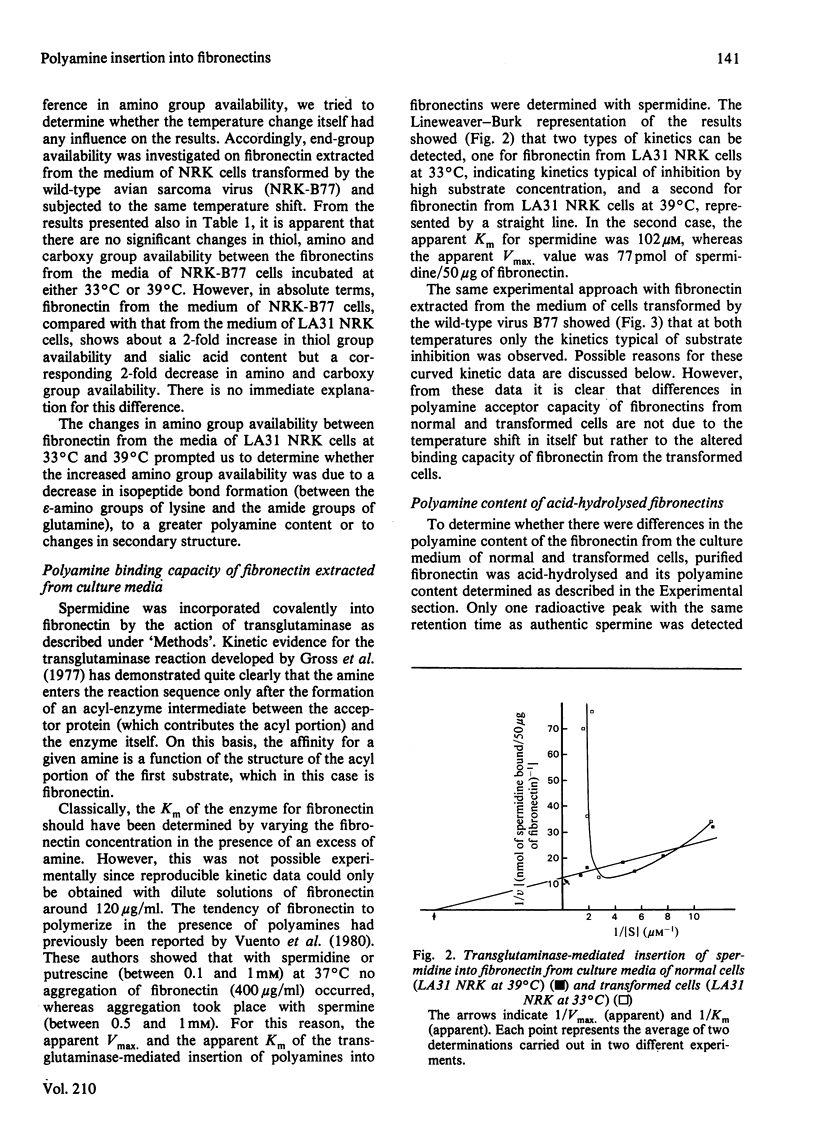
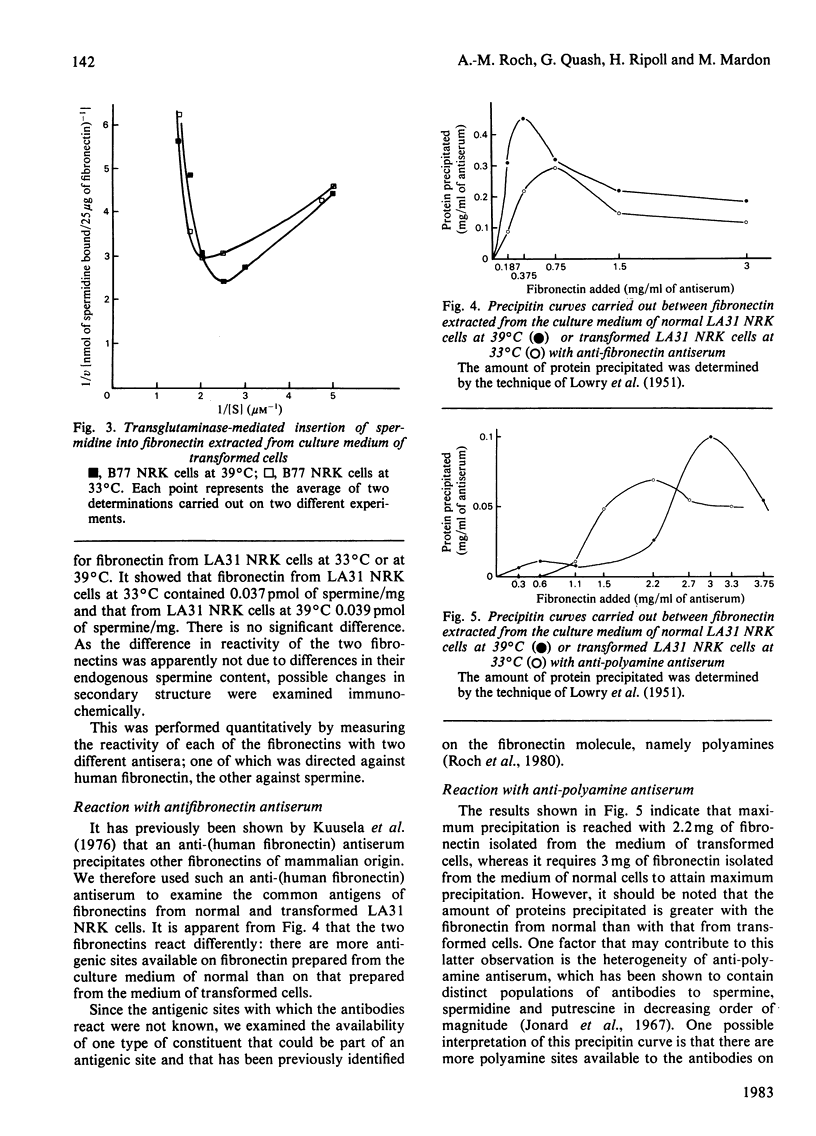
Abstract
1. Fibronectin released from transformed rat kidney cells compared with that released from normal rat kidney cells shows a 50% increase in amino group availability. 2. No such changes were observed in thiol and carboxy group availability or in sialic acid content. 3. The increased amino group availability is not due to a greater polyamine content, which was about 0.04 pmol/mg of protein. 4. Transglutaminase mediated the insertion of spermidine into normal cell fibronectin with linear kinetics. With fibronectin from transformed cells (temperature-sensitive mutant or wild-type), kinetics typical of substrate inhibition were observed. 5. Immunochemical analysis with an anti-polyamine antiserum and an anti-(human fibronectin) antiserum showed that fibronectins from normal and transformed cells react differently. The significance of these results is discussed in the light of changes in the secondary structure between the two fibronectins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali I. U., Hunter T. Structural comparison of fibronectins from normal and transformed cells. J Biol Chem. 1981 Jul 25;256(14):7671–7677. [PubMed] [Google Scholar]
- Bartos D., Bartos F., Campbell R. A., Grettie D. P., Smejtek P. Antibody to spermine: a natural biological constituent. Science. 1980 Jun 6;208(4448):1178–1181. doi: 10.1126/science.7375929. [DOI] [PubMed] [Google Scholar]
- Birckbichler P. J., Orr G. R., Patterson M. K., Jr Differential transglutaminase distribution in normal rat liver and rat hepatoma. Cancer Res. 1976 Aug;36(8):2911–2914. [PubMed] [Google Scholar]
- Chen Y. C., Hayman M. J., Vogt P. K. Properties of mammalian cells transformed by temperature-sensitive mutants of avian sarcoma virus. Cell. 1977 Jul;11(3):513–521. doi: 10.1016/0092-8674(77)90069-1. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Folk J. E., Park M. H., Chung S. I., Schrode J., Lester E. P., Cooper H. L. Polyamines as physiological substrates for transglutaminases. J Biol Chem. 1980 Apr 25;255(8):3695–3700. [PubMed] [Google Scholar]
- Furuichi K., Ezoe H., Obara T., Oka T. Evidence for a naturally occurring anti-spermine antibody in normal rabbit serum. Proc Natl Acad Sci U S A. 1980 May;77(5):2904–2908. doi: 10.1073/pnas.77.5.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODFRIEND T. L., LEVINE L., FASMAN G. D. ANTIBODIES TO BRADYKININ AND ANGIOTENSIN: A USE OF CARBODIIMIDES IN IMMUNOLOGY. Science. 1964 Jun 12;144(3624):1344–1346. doi: 10.1126/science.144.3624.1344. [DOI] [PubMed] [Google Scholar]
- Gross M., Whetzel N. K., Folk J. E. Amine binding sites in acyl intermediates of transglutaminases. Human blood plasma enzyme (activated coagulation factor XIII) and guinea pig liver enzyme. J Biol Chem. 1977 Jun 10;252(11):3752–3759. [PubMed] [Google Scholar]
- Habeeb A. F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966 Mar;14(3):328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- Heby O. Role of polyamines in the control of cell proliferation and differentiation. Differentiation. 1981;19(1):1–20. doi: 10.1111/j.1432-0436.1981.tb01123.x. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Cell surface proteins and malignant transformation. Biochim Biophys Acta. 1976 Apr 30;458(1):73–107. doi: 10.1016/0304-419x(76)90015-9. [DOI] [PubMed] [Google Scholar]
- Jonard J., Quash G., Wahl R. La spécificité des anticorps antipolyamines. C R Acad Sci Hebd Seances Acad Sci D. 1967 Oct 9;265(15):1099–1102. [PubMed] [Google Scholar]
- Jänne J., Pösö H., Raina A. Polyamines in rapid growth and cancer. Biochim Biophys Acta. 1978 Apr 6;473(3-4):241–293. doi: 10.1016/0304-419x(78)90015-x. [DOI] [PubMed] [Google Scholar]
- Kuusela P., Ruoslahti E., Engvall E., Vaheri A. Immunological interspecies cross-reactions of fibroblast surface antigen (fibronectin). Immunochemistry. 1976 Aug;13(8):639–642. doi: 10.1016/0019-2791(76)90203-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levitzki A., Willingham M., Pastan I. Evidence for participation of transglutaminase in receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1980 May;77(5):2706–2710. doi: 10.1073/pnas.77.5.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorand L., Parameswaran K. N., Stenberg P., Tong Y. S., Velasco P. T., Jönsson N. A., Mikiver L., Moses P. Specificity of guinea pig liver transglutaminase for amine substrates. Biochemistry. 1979 May 1;18(9):1756–1765. doi: 10.1021/bi00576a019. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Chen A. B., Huseby R. M. The cold-insoluble globulin of human plasma: studies of its essential structural features. Biochim Biophys Acta. 1975 Apr 29;386(2):509–524. doi: 10.1016/0005-2795(75)90294-9. [DOI] [PubMed] [Google Scholar]
- Mosher D. F. Action of fibrin-stabilizing factor on cold-insoluble globulin and alpha2-macroglobulin in clotting plasma. J Biol Chem. 1976 Mar 25;251(6):1639–1645. [PubMed] [Google Scholar]
- Olden K., Yamada K. M. Mechanism of the decrease in the major cell surface protein of chick embryo fibroblasts after transformation. Cell. 1977 Aug;11(4):957–969. doi: 10.1016/0092-8674(77)90307-5. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Strominger J. L. Transglutaminase modifies the carboxy-terminal intracellular region of HLA-A and -B antigens. Nature. 1981 Feb 26;289(5800):819–821. doi: 10.1038/289819a0. [DOI] [PubMed] [Google Scholar]
- Quash G., Delain E., Huppert J. Effect of antipolyamine antibodies on mammalian cells in tissue culture. Exp Cell Res. 1971 Jun;66(2):426–432. doi: 10.1016/0014-4827(71)90697-5. [DOI] [PubMed] [Google Scholar]
- Quash G., Jonard J. Immunochimie des polyamines. C R Acad Sci Hebd Seances Acad Sci D. 1967 Sep 25;265(13):934–936. [PubMed] [Google Scholar]
- Quash G., Roch A. M. Les polyamines chex les eucaryotes: les mécanismes homéostatiques intracellulaires et sériques. Ann Biol Clin (Paris) 1979;37(6):317–325. [PubMed] [Google Scholar]
- Roch A. M., Quash G., Huppert J. Mise en évidence dans les sérums humains d'anticorps (IgG) réagissant spécifiquement avec les polyamines. C R Acad Sci Hebd Seances Acad Sci D. 1978 Oct 30;287(11):1071–1074. [PubMed] [Google Scholar]
- Roch A. M., Quash G., Huppert J. Mise en évidence immunochimique de l'attachement spécifique de la putrescine aux proteines plasmatiques dont la fibronectine. C R Seances Acad Sci D. 1980 Feb 11;290(6):449–452. [PubMed] [Google Scholar]
- Schrode J., Folk J. E. Transglutaminase-catalyzed cross-linking through diamines and polyamines. J Biol Chem. 1978 Jul 25;253(14):4837–4840. [PubMed] [Google Scholar]
- Teng M. H., Rifkin D. B. Fibronectin from chicken embryo fibroblasts contains covalently bound phosphate. J Cell Biol. 1979 Mar;80(3):784–791. doi: 10.1083/jcb.80.3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaheri A., Mosher D. F. High molecular weight, cell surface-associated glycoprotein (fibronectin) lost in malignant transformation. Biochim Biophys Acta. 1978 Sep 18;516(1):1–25. doi: 10.1016/0304-419x(78)90002-1. [DOI] [PubMed] [Google Scholar]
- Vuento M., Vaheri A. Dissociation of fibronectin from gelatin-agarose by amino compounds. Biochem J. 1978 Oct 1;175(1):333–336. doi: 10.1042/bj1750333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuento M., Vartio T., Saraste M., von Bonsdorff C. H., Vaheri A. Spontaneous and polyamine-induced formation of filamentous polymers from soluble fibronectin. Eur J Biochem. 1980 Mar;105(1):33–42. doi: 10.1111/j.1432-1033.1980.tb04471.x. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Wilson B. S., Ruberto G., Ferrone S. Sulfation and molecular weight of fibronectin shed by human melanoma cells. Biochem Biophys Res Commun. 1981 Aug 14;101(3):1047–1051. doi: 10.1016/0006-291x(81)91854-4. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Schlesinger D. H., Kennedy D. W., Pastan I. Characterization of a major fibroblast cell surface glycoprotein. Biochemistry. 1977 Dec 13;16(25):5552–5559. doi: 10.1021/bi00644a025. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Gabbay M., Schlessinger J. Primary amines do not prevent the endocytosis of epidermal growth factor into 3T3 fibroblasts. Biochim Biophys Acta. 1981 May 5;674(2):188–203. doi: 10.1016/0304-4165(81)90377-9. [DOI] [PubMed] [Google Scholar]