Abstract
Prenatal stress exerts long-term impact on neurodevelopment in the offspring, with consequences such as increasing the offspring’s risk of depression in adolescence and early adulthood. S-ketamine can produce rapid and robust antidepressant effects, but it is not clear yet whether and how S-ketamine alleviates depression in prenatally stressed offspring. The current study incestigated the preliminary anti-depression mechanism of S-ketamine in prenatally stressed offspring, particularly with regard to neuroplasticity. The pregnant females were given chronic unpredictable mild stress on the 7th-20th day of pregnancy and their male offspring were intraperitoneally injected with a single dose of S-ketamine (10 mg/kg) on postnatal day 42. Our findings showed that S-ketamine treatment counteracted the development of depression-like behaviors in prenatally stressed offspring. At the cellular level, S-ketamine markedly enhanced neuroplasticity in the CA1 hippocampus: Golgi-Cox staining showed that S-ketamine alleviated the reduction of neuronal complexity and dendritic spine density; Transmission electron microscopy indicated that S-ketamine reversed synaptic morphology alterations. At the molecular level, by western blot and RT-PCR we detected that S-ketamine significantly upregulated the expression of BDNF and PSD95 and activated AKT and mTOR in the hippocampus. In conclusion, prenatal stress induced by chronic unpredictable mild stress leads to depressive-like behaviors and hippocampal neuroplasticity impairments in male offspring. S-ketamine can produce antidepressant effects by enhancing hippocampal neuroplasticity via the BDNF/AKT/mTOR signaling pathway.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-76226-y.
Keywords: Prenatal stress, Offspring, S-ketamine, Depression, Hippocampus, Neuroplasticity
Subject terms: Depression, Molecular biology, Experimental models of disease
Introduction
Gestation is one of the most complex and vulnerable periods during a woman’s life and pregnant women are highly sensitive to a variety of external stimuli. According to the fetal programming hypothesis, an unfavorable in utero environment can affect infant development and cause sustained deleterious effects on offspring stress response1. Prenatal stress (PNS) refers to the stress during pregnancy, including stressful life events, poor nutrition, illness, adverse environmental factors, etc2. Clinical and animal studies have shown that PNS can exert long-term adverse impacts on the central nervous system in offspring, thus increasing the offspring’s risk of depression in adolescence and early adulthood3–6. There are multiple approaches to induce PNS in animal models7–10. As is well known, the chronic unpredictable mild stress (CUMS) model, a well-validated and widely used animal model of depression, closely mimics the social stressors suffered by human beings11,12. Likewise, maternal exposure to CUMS during rodent pregnancy can largely simulate exposure of pregnant women to daily life environmental challenges.
Depression is a common psychiatric illness, characterized by persistently depressed mood, anhedonia, loss of interest and low self-esteem and energy13. Numerous studies14–18 show that depression is related to abnormalities in the prefrontal cortex, hippocampus, and amygdala and is accompanied by a volume reduction of brain tissue, neurite atrophy and synapse loss in these brain regions. The hippocampus is one of the most stress-susceptible and plastic regions in the brain19 and it seems that improving structure and function of the hippocampus could be considered a very promising target for treating depression.
The N-methyl-D-aspartate receptor (NMDAR) antagonist (R, S)-ketamine (ketamine) is a racemic mixture comprising equal parts of R-ketamine and S-ketamine. Traditional antidepressant drugs such as selective serotonin reuptake inhibitors (SSRIs) or monoamine oxidase inhibitors (MAOIs) have slower onset, longer treatment time and limited efficacy for treatment-resistant depression20. Ketamine differs from traditional antidepressant drugs by providing effective and fast-acting treatment for patients with depression21–24. On 5 March 2019, S-ketamine was approved by the Food and Drug Administration as a rapid-acting antidepressant in conjunction with an oral antidepression for the management of treatment-resistant depression in adults25. There is a great deal of disagreement about the mechanism of ketamine in depression treatment, but the vast majority of theories propose that changes in neuroplasticity play a critical role26–29. The concept of neuroplasticity is the ability of the nervous system to adapt and respond to the environment30, which depends on healthy levels of neurotrophic factors, dendritic complexity, neurogenesis synaptogenesis and glial cell function31. Among them, synapses are the key structures involved in signal transduction and neuroplasticity32. Increased expression and release of brain-derived neurotrophic factor (BDNF) and activation of the mammalian target of rapamycin (mTOR) are components known to be associated with synapse formation and synaptic plasticity33. Previous studies have indicated that S-ketamine exerts its anti-depressive effect by increasing hippocampal synaptic plasticity in multiple rodent models of depression33–35. However, whether and how S-ketamine reverses offspring depression induced by PNS remains poorly understood.
The mTOR is a serine/threonine protein kinase that has been shown to play a fundamental role in mammalian neurogenesis, particularly in axon and dendrite development and synaptic plasticity36. Evidence suggests that the mTOR pathway is tightly associated with the pathogenesis and treatment of depression37. Structurally, mTOR exists as two complexes: mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). Activation of AKT (Protein kinase B) activates mTORC1 and the activation of mTORC1 leads to phosphorylation of both eukaryotic translation initiation factor ribosomal protein S6 kinase beta-1 (S6K1) and 4E-binding protein 1 (4E-BP1). S6K1 and 4E-BP1 promote protein translation and cell growth by stimulating ribosomal protein S6 phosphorylation and releasing the translation initiation factor eIF4E38. As de novo protein synthesis is crucial for synaptic plasticity and dendritic spine maintenance36, mTORC1 plays an essential role in learning, memory and cognition. Overexpression of synaptic proteins such as synaptophysin (SYN) and post-synaptic density protein 95 (PSD95) may be involved in the formation, maturation, and function of new synapses39.
BDNF is a member of neurotrophin family and it has important regulatory effect on neuronal growth, maturation, differentiation and maintenance40. BDNF binding to its cognate receptor tropomyosin receptor kinase B (TrkB) triggers multiple intracellular signaling cascades, including those regulated by the mTORC1, which in turn induce protein synthesis and dendrite outgrowth41.
In this study, we established a prenatal CUMS model to study the effects of S-ketamine on depression in PNS offspring. We proposed that S-ketamine might alleviate depression-like behavior and enhance hippocampal neuroplasticity in PNS offspring via the BDNF/AKT/mTOR signaling pathway.
Materials and methods
Animals and breeding
8–9 weeks virgin female and fertile male C57/BL6J mice were obtained from GemPharmatech (Jiangsu, China). The mice were fed standard diet ad libitum and housed under conventional conditions at a controlled temperature (24 ± 1 °C), humidity (50% ± 2%) and lighting (12 h light/12 h darkness, lights on at 8:00 am and off at 8:00 pm). Breeding and Modeling were carried out 1 week after adaptive feeding.
Female mice were mated with male mice overnight at a ratio of 2:1 and then checked for vaginal plugs at 8:00 am in the following day. The day of vaginal plug detection was defined as the gestational day (GD) 1 and the first postnatal day of the pups was defined as postnatal day (PND) 1. On GD1, pregnant females were randomly assigned to control and PNS groups. On PND1, the litter were culled randomly, leaving eight pups per litter. Pregnant females were housed individually with pups until weaning on PND21. Only male offspring were used in all of our studies. To avoid potential litter effect, a maximum of one male offspring per litter was used in each experiment.
The study was performed in accordance with ARRIVE guidelines. The study was approved by the Anhui Medical University (No. LLSC20170331). All methods were performed in accordance with the relevant guidelines and regulations.
PNS paradigm
The PNS group of pregnant females were subjected to CUMS procedure from GD7 to GD20 according to previously described methods4. The selection of the daily stress type was based on the simple random sampling method (Table 1).
Table 1.
The two-week CUMS protocol.
| Day | Monday | Tuesday | Wednesday | Thursday | Friday | Saturday | Sunday |
|---|---|---|---|---|---|---|---|
| GD7-GD13 |
Restraint (2 h) |
Water deprivation (24 h) |
Night-lights (12 h) |
Cold swimming (5 min) |
Warm swimming (15 min) |
Food deprivation (24 h) |
Tail pinch (5 min) |
| GD14-GD20 |
Water deprivation (24 h) |
Warm swimming (15 min) |
Tail pinch (5 min) |
Cold swimming (5 min) |
Restraint (2 h) |
Night-lights (12 h) |
Food deprivation (24 h) |
Drug administration
S-ketamine was purchased from Jiangsu Heng Rui Medicine Co. Ltd. (50 mg/2 ml, Jiangsu, China) and was diluted in 0.9% saline before usage. Single-dose injections were given, each with a volume of 10 mg/kg. The dosage of ketamine was based on previous literature42.
Experimental design
Pregnant females were assigned randomly to two groups of sixteen each—a control group and a PNS group. At 12:00 am on PND42, male offspring were intraperitoneally (IP) injected with S-ketamine or saline and behavioral tests were performed 1 h later. Hence there were four experimental offspring groups, based on combinations of PNS paradigm and drug treatment: Control + Saline, Control + S-ketamine, PNS + Saline, PNS + S-ketamine. Each group contained sixteen male offspring from eight litters.
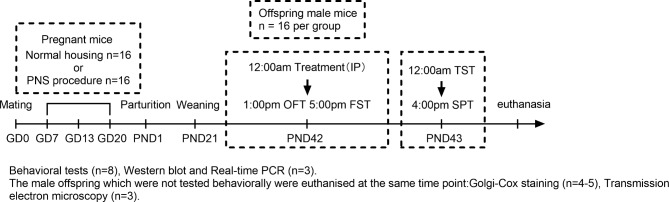
Groups of eight male offspring from eight litters performed all the behavioral tests. Immediately after the behavioral tests, the offspring were euthanized by an intraperitoneal injection of 50 mg/kg pentobarbital, followed by transcardial perfusion with physiological saline. After evaluating the behaviour of each group, 3 mice from each group were immediately used for perform western blot and Real-time PCR. Both sides of the hippocampus were collected to perform western blot and Real-time PCR analysis, respectively. The remaining 5 male offspring in each group were used for Nissl Staining. The male offspring which were not tested behaviorally were euthanised at the same time point and assigned to Golgi-Cox staining (4–5 mice in each group) and Transmission electron microscopy (3 mice in each group). The experimental timeline is shown in Fig. 1.
Fig. 1.
The experimental timeline. OFT, open field test; FST, forced swim test; TST, tail suspension test; SPT, sucrose preference test.
Behavioral tests
One hour after injection, behavioral tests were conducted to verify behavioral distinctions of different groups. Except for the Sucrose preference test, we recorded all the behaviors of mice with an automated video tracking system (Shanghai Xinruan Information Technology Co., Ltd., Shanghai, China). Eight mice from each group were subjected to the behavioral tests.
Open field test (OFT)
The OFT was conducted to evaluate the locomotor activity of the mice through measurements of total traveled distance. Each mouse was placed in an experimental cage (length × width × height: 500 × 500 × 400 mm) and allowed to move freely in the dim lighting for 5 min. Experimental apparatus was carefully cleaned with 75% alcohol after the test of each mouse.
Forced swim test (FST)
The FST test was carried out to measure the degree of behavioral despair in offspring. Each mouse was placed individually in a clear plastic cylinder (diameter: 10 cm; height: 25 cm) containing 18 cm of water, maintained at 24 ± 1 °C. Immobility was defined as the cessation of limb movements except slight involuntary hind limb movements43. Each mouse was kept in the apparatus for a session of 6 min and the total time it spent immobile during the trial was recorded. Water in the container was changed after each test.
Tail suspension test (TST)
As another test for evaluation of the degree of behavioral despair, the mice were individually suspended 50 cm above a solid surface by attaching their tails to a horizontal metal bar using adhesive tape. The total amount of time each mouse spent immobile (mice were considered immobile only when they hung passively without any movement of the head or paws44 during a 6-min period was recorded as the immobility time.
Sucrose preference test (SPT)
SPT was carried out as described previously45 in order to assess anhedonia levels. Mice were given ad libitum access to one bottle with water and one bottle with 1% sucrose water for 48 h, and the positions of two bottles were exchanged each 6 h. After 4 h of water and food deprivation, 2 identical bottles (one containing water and the other containing 1% sucrose water) were put into each cage, and the sucrose preference test began. The bottles were left for 1 h and weighted before and after testing to measure the intake from each bottle. The sucrose preference was calculated as a percentage of sucrose solution consumption to the total liquid consumption during the 1 h test.
Golgi-Cox staining
Anesthetized mice were perfused with physiological saline, and their brains were then dissected out and placed in impregnation solution (1% potassium dichromate, 1% mercuric chloride and 0.8% potassium chromate). After 12 h, the brains were placed in new impregnation solution, and then stored at room temperature in the dark for one week. Afterwards, the brains were transferred to 30% sucrose solution for one day. Thereafter, the brains were cut into slices of 100 μm thickness on a 7000 smz-2 vibratome (Campden) and mounted on 5% gelatin-coated microscope slides. After being air-dried overnight, the slices had 75% ammonia solution and 1% sodium thiosulfate applied to them. Finally, the slices were dehydrated in an alcohol gradient, cleared with xylene and covered with resinous mounting medium. Sholl analysis and dendritic spine density analysis were adopted to analyze the morphological changes of Golgi impregnated CA1 pyramidal neurons46. The images of CA1 pyramidal neurons were taken under microscope (×200) at the same position and different focal distances (one image every 4 μm in the z axis). The images were synthesized with the NeuronStudio software package, version 0.9.92 (http://research.mssm.edu/cnic/tools.html). For each selected neuron, all branches of the dendritic tree were manually reconstructed using Fiji Image J version 1.53c (https://imagej.net/Fiji). For each group, 8 neurons from 4 mice. This was done to refer to previous research46, Sholl analysis was performed at 10 μm intervals to quantify both apical and basal dendritic arborization using the Sholl plug-in. Number of spines per 10 μm was manually counted using a 100x oil immersion lens. Spines were counted on secondary branches of basal dendrites in the CA1 hippocampal region. From 5 branches for each mouse, and 5 mice per group were analyzed. For each group, average spine density of each mice analyzed was averaged to arrive at the spine density used for statistical analysis.
Transmission electron microscopy (TEM)
Mice were anesthetized and then perfused with physiological saline followed by 2.5% glutaraldehyde. Brains were rapidly removed on ice and a 1 mm³ tissue sample was cut from the hippocampal CA1 area. The samples were washed with 0.01 M PBS for 3 times, post-fixed in 1% osmium tetroxide at room temperature for 2 h, and washed again 3 times with PBS. The samples were dehydrated in gradient alcohol and 100% acetone, and then embedded in EPON 812 epoxy resin. Ultrathin slices (60–80 nm) were cut with a Leica EM UC7 ultramicrotome and stained with 2% uranium acetate saturated alcohol solution and lead citrate. These slices were observed by a transmission electron microscope (HT7700, Japan). The thickness of the postsynaptic density and the width of the synaptic cleft were quantified using ImageJ, in accordance with previous methods47,48. The thickness of the postsynaptic density was evaluated as the length of the perpendicular line from the postsynaptic membrane to the most convex part of the synaptic complex. The width of the synaptic cleft was estimated by measuring the narrowest and widest portions of the synapse and then taking the average of these values. 8 synapses form 3 CA1 tissue per group were selected for ultrastructure analysis.
Western blot (WB) analysis
Protein extraction and quantification were performed according to previously described methods49. The hippocampal tissues were stored at − 80 °C until experimental use. The hippocampus was minced into homogenates in 300ul lysis buffer with 1% protease and 2% phosphatase inhibitors. The supernatants were aspirated after the homogenates were centrifuged at 4 °C at 15,000 rpm for 15 min. Protein content was determined with BCA assay and samples diluted to 500 ng protein per µL. Proteins (20 µg) were separated by 8–12% SDS-PAGE and subsequently transferred to PVDF membrane. The membrane was blocked with 5% nonfat dry milk (2 h, Room temperature), followed by incubation with primary antibodies (overnight, 4 °C) and secondary antibodies (1 h, Room temperature). The primary antibodies chosen in this study were: AKT (1:10000; Abcam), p-AKT (1:1000; Abmart), mTOR (1:1000; Abmart), p-mTOR (1:1000; Abmart), BDNF (1:500; Proteintech), PSD-95 (1:1000; Proteintech), SYN (1:5000; Proteintech), β-actin (1:5000; Proteintech). Goat anti-rabbit and goat anti-mouse second antibody were purchased from ZSGB-BIO and were used in a 1:10,000 dilution. Appropriate secondary antibodies were chosen according to the primary antibodies. Proteins were visualized using enhanced chemiluminescence (ECL) and ChemiDoc MP Imaging System (Bio-Rad). Optical density analysis was performed using ImageJ and normalized by β-actin protein levels. Furthermore, phospho-protein signals were normalized to their total protein levels.
Real-time PCR (RT-PCR) analysis
Total RNA was extracted from hippocampal tissues using Trizol reagent (Invitrogen). The RNA was eluted with 40 µL RNase-free H2O and concentration and purity was measured by NanoDrop spectrophotometer. The qualified RNA concentration was adjusted to 250ng/µL with RNase-free water. According to the requirements of the instructions, the SPARKscript II RT Plus kit (Sparkjade) was used to convert RNA into cDNA to avoid RNA degradation. The cDNA samples were stored at -80 °C until use. RT-qPCR was performed using 2×SYBR Green qPCR Mix (Sparkjade). Each cDNA sample had two independent replicates performed to ensure its reliability. Primer sequences for RT-PCR are shown in Table 2. The relative expression levels of target genes were normalized against β-Actin and calculated by 2-(ΔΔCt) method.
Table 2.
The primer sequences of each gene.
| Gene | Forward primer(5’→3’) | Reverse primer(5’→3’) |
|---|---|---|
| BDNF | TGTGACAGTATTAGCGAGTGGGT | CGATTGGGTAGTTCGGCATT |
| PSD95 | TCTGTGCGAGAGGTAGCAGA | AAGCACTCCGTGAACTCCTG |
| Synaptophysin | TCTTTGTCACCGTGGCTGTGTT | TCCCTCAGTTCCTTGCATGTGT |
| β-actin | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
Statistical analysis
Sholl analysis data were represented as mean ± standard error of the mean (SEM), other quantitative data were shown as mean ± standard deviation (SD). Data were statistically analyzed with SPSS version 22.0 (Chicago, IL, USA). All graphs were constructed in GraphPad Prism 9 (GraphPad, San Diego, CA, USA). Prior to statistical analysis, normality of data (Shapiro-Wilk test) and homogeneity of variances (Levene’s test) were checked. Differences between groups were analyzed using two-way ANOVA and a difference was considered significant when P < 0.05. Significant main effects and interactions were further investigated with Bonferroni post hoc tests. Details for the statistical analyses can be found in the Supporting Information.
Results
S-ketamine ameliorated PNS-induced depression-like behavior in offspring
In the OFT, no significant main effects and interactions between PNS paradigm and drug treatment for total traveled distance within 5 min was observed (Fig. 2A), suggesting that neither the PNS paradigm nor the S-ketamine administration can influence the locomotor activity of mice. On the other hand, compared with Control + Saline mice, PNS + Saline mice displayed significantly increased immobility time in both FST (Fig. 2B, P < 0.01) and TST (Fig. 2C, P < 0.001), while S-ketamine significantly reversed the increased immobility time for both FST (P < 0.01) and TST (P < 0.01). In the SPT, PNS paradigm decreased the sucrose preference (Fig. 2D, P < 0.01), which was reversed by S-ketamine treatment (P < 0.05). These findings suggested that S-ketamine treatment can alleviate the anhedonia and despair behavior caused by PNS paradigm.
Fig. 2.
S-ketamine administration rescued PNS-induced depression-like behaviors. (A) The total distance in the open field. (B) Immobility time in the forced swim test (FST). (C) Immobility time in the tail suspension test (TST). (D) Sucrose consumption percentage in SPT. Data are presented as mean ± SEM (n = 8 mice per group). **P < 0.01, ***P < 0.001 PNS + Saline versus Control + Saline; #P < 0.05, ##P < 0.01 PNS + S-ketamine versus PNS + Saline. NS indicates no statistical significance.
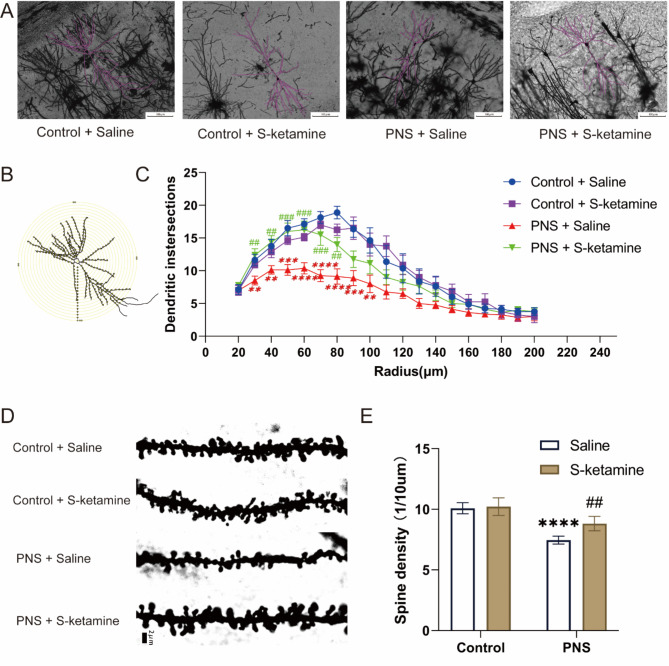
S-ketamine alleviated PNS-induced reduction of neuronal complexity and dendritic spine density in hippocampus
To elucidate the effect of PNS and S-ketamine on the hippocampal structural plasticity, we adopted Sholl analysis and spine count. Representative images are shown in Fig. 3A, B, D. Compared with the Control + Saline group, the PNS + Saline group showed a lower density of dendritic spines (Fig. 3E, P < 0.0001) and a significantly decreased number of dendritic intersections in the circle diameter of 30–90 μm (Fig. 3C, 30 μm: P < 0.01; 40 μm: P < 0.01; 50 μm: P < 0.001; 60 μm: P < 0.0001; 70 μm: P < 0.0001; 80 μm: P < 0.0001; 90 μm: P < 0.001; 100 μm: P < 0.01); Compared with the PNS + Saline group, treatment with S-ketamine significantly increased the density of dendritic spines (P < 0.01) and the number of intersections between 30 and 80 μm (30 μm: P < 0.01; 40 μm: P < 0.01; 50 μm: P < 0.001; 60 μm: P < 0.001; 70 μm: P < 0.001; 80 μm: P < 0.01).
Fig. 3.
Structural changes in hippocampal CA1 neurons were elucidated by Golgi staining. (A) Representative images of reconstructed hippocampal CA1 neurons in each group (magnification 400 ×, Scale bar = 200 μm). (B) A schematic diagram of CA1 neurons with concentric circles used for the Sholl analysis. (C) Sholl analysis of the number of intersections in CA1 hippocampal pyramidal neurons in each group. The radius interval between the circles was 10 μm per step, ranging from 20 μm to 200 μm from the center of the neuronal soma. Data are presented as mean ± SEM (n = 8 neurons from 4 mice for each group). (D) Representative images of dendritic spines on secondary branches from hippocampal CA1 neurons (magnification 1000 ×, Scale bar = 2 μm). (E) Quantitative analysis of dendritic spine density. Data are presented as mean ± SD (n = 5 mice per group, and 5 branches per mouse were analyzed). **P < 0.01, ***P < 0.001, ****P < 0.0001, PNS + Saline versus Control + Saline; ##P < 0.01, ###P < 0.001, PNS + S-ketamine versus PNS + Saline.
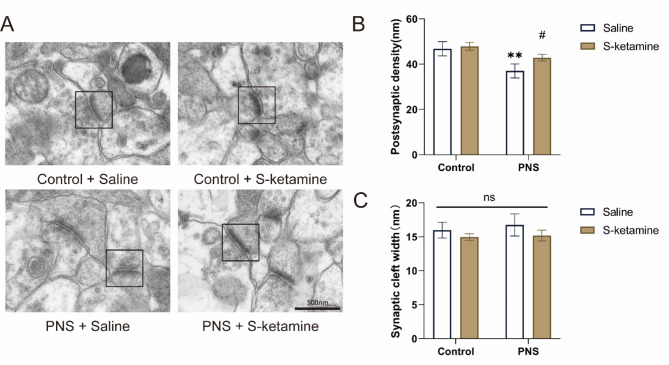
S-ketamine corrected the ultrastructural morphological changes of synapses in hippocampus
Representative images of synaptic ultrastructure in CA1 in each group are shown in Fig. 4A. Compared with the Control + Saline group, the thickness of postsynaptic density of the PNS + Saline group was significantly reduced (Fig. 4B, P < 0.01). Treatment with S-ketamine increased the thickness of postsynaptic density compared with the PNS + Saline group (P < 0.05). There were no significant differences in the synaptic space among all four groups (Fig. 4C, P > 0.05).
Fig. 4.
Effects of PNS exposure and S-ketamine on the ultrastructure of synapse in offspring. (A) Representative images of synaptic ultrastructure in CA1 in each group (magnification 20,000 ×, Scale bar = 500 nm). (B) Quantitative analysis of postsynaptic density thickness. (C) Quantitative analysis of synaptic cleft width. Data are presented as mean ± SD. **P < 0.01, PNS + Saline versus Control + Saline; #P < 0.05, PNS + S-ketamine versus PNS + Saline. Data are presented as mean ± SD (n = 3 mice per group, 8 synapses per mouse). NS indicates no statistical significance.
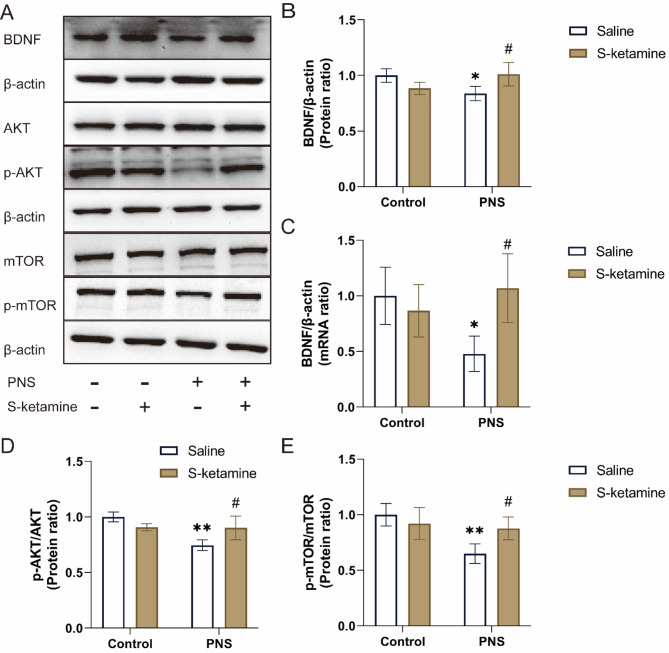
S-ketamine mediated the activation of BDNF/AKT/mTOR signaling pathway in hippocampus
BDNF is a crucial factor that promotes neuronal plasticity, and the neuroplasticity role of BDNF can be mediated by the AKT/mTOR signaling pathway41. Accordingly, we examined BDNF hippocampal expression using RT-PCR and western blot analyses. Representative western blot image of BDNF, AKT, p-AKT, mTOR and p-mTOR in the hippocampus are shown in Fig. 5A. Prenatal stress significantly decreased both mRNA and protein levels of BDNF (Fig. 5B, C, P < 0.05). S-ketamine significantly elevated the protein and mRNA levels of BDNF compared with the PNS + Saline group (P < 0.05). In addition, we tested the AKT and mTOR activity (Fig. 5D, E). Compared with the Control + Saline group, the ratios of the p-AKT/AKT and p-mTOR/mTOR in the PNS + Saline group were significantly decreased (P < 0.01). Compared with the PNS + Saline group, treatment with S-ketamine increased the p-AKT/AKT and p-mTOR/mTOR ratios (P < 0.05).
Fig. 5.
Effects of PNS exposure and S-ketamine on the BDNF/AKT/mTOR signaling pathway. (A) Representative western blot image of BDNF, AKT, p-AKT, mTOR and p-mTOR in the hippocampus. (B) BDNF protein levels were quantified by densitometry analysis and normalized to β-actin. (C) Levels of BDNF mRNA were normalized by β-actin mRNA. (D, E) Densitometric analysis of the ratios of p-mTOR/mTOR and p-AKT/AKT. Data are presented as mean ± SD (n = 3 mice per group). *P < 0.05, **P < 0.01, PNS + Saline versus Control + Saline; #P < 0.05, PNS + S-ketamine versus PNS + Saline.
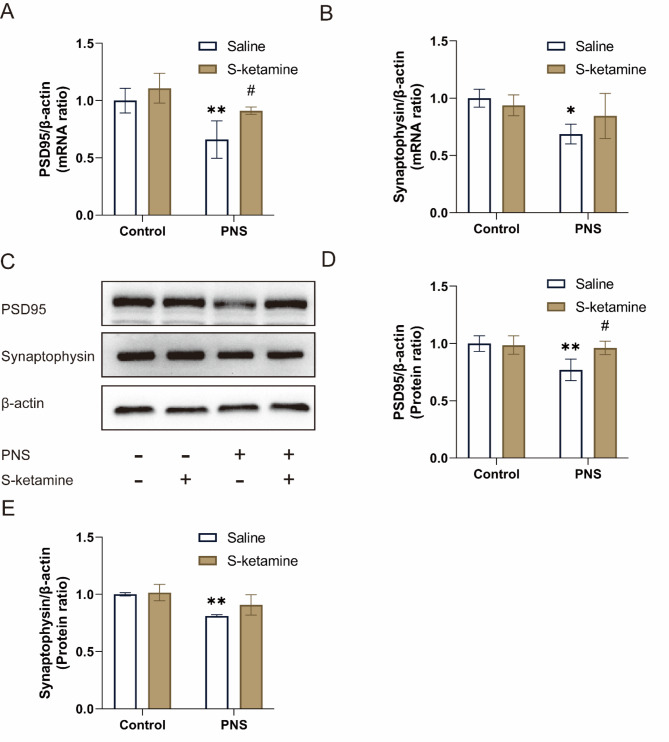
S-ketamine regulated the expression of synaptic proteins in hippocampus
As shown in Fig. 6A, B, we analyzed the mRNA expression levels of synaptic proteins (synaptophysin and PSD95) in the hippocampus of the offspring. Compared with the Control + Saline group, the mRNA expressions of PSD95 and synaptophysin in the PNS + Saline group were significantly decreased (P < 0.01, P < 0.05, respectively). Compared with the PNS + Saline group, treatment with S-ketamine significantly increased the PSD95 mRNA level (P < 0.05). However, there is no significant difference in the mRNA level of synaptophysin between the PNS + Saline group and the PNS + S-ketamine group (P > 0.05). Further quantification by Western blot showed similar results (Fig. 6D, E). Representative western blot image of PSD95 and synaptophysin in the hippocampus are shown in Fig. 6C. Prenatal stress significantly decreased the protein levels of PSD95 and synaptophysin (P < 0.01). After treatment with S-ketamine, the PSD95 protein level increased (P < 0.05). No significant difference was observed in the protein level of synaptophysin between the PNS + Saline group and the PNS + S-ketamine group (P > 0.05).
Fig. 6.
Effects of PNS exposure and S-ketamine on the expression of synaptic proteins. (A, B) Levels of PSD95 and synaptophysin mRNA were normalized by β-actin mRNA. (C) Representative western blot image of PSD95 and synaptophysin in the hippocampus. (D, E) PSD95 and synaptophysin protein levels were quantified by densitometry analysis and normalized to β-actin. Data are presented as mean ± SD (n = 3 mice per group). *P < 0.05, **P < 0.01, PNS + Saline versus Control + Saline; #P < 0.05, PNS + S-ketamine versus PNS + Saline.
Discussion
Depression is the leading cause of social burden and disability-adjusted life years worldwide50. Due to the increasingly competitive nature of the social environment, pregnant women are frequently exposed to stressful life events. Since depression is a multifactorial disorder that involves genetic and environmental factors7,26,51 and children exposed to maternal depression are more likely to develop treatment-resistant depression52, it is important to investigate the relationship between early life exposures and offspring depression. At the same time, there is an urgent need to identify corresponding therapeutic strategies. Ketamine is a promising drug for the treatment of depression and represents a new frontier for psychiatric research. When administered as a single subanesthetic dose, ketamine could produce rapid (within hours) and relatively long-term antidepressant actions even in patients with major depression33. Ketamine of sub-anesthetic doses has been shown to induce neuroplastic changes over periods of hours to days following exposure in rodent models53,54. In this study, we established a prenatal CUMS model to investigate the regulatory mechanism of S-ketamine in offspring depression.
The male offspring which were exposed to prenatal stress exhibited depression-like behavior, confirming previous reports5,51,55. This behavior was manifested through increased immobility time in both FST and TST and decreased sucrose preference in SPT, suggesting behavioral despair and anhedonia. This suggested that the PNS-induced depression model was successfully established. Furthermore, a single dose of S-ketamine was found to indeed reverse the long-term effects of prenatal stress in offspring.
In recent years, people have gradually realized that chronic stress negatively affects neural plasticity and neurogenesis, which consequently produce depressive symptoms, as well as impairment in memory and learning processes56. Many published studies have provided clear evidence of neuronal atrophy and decreases synaptic density in CUMS depression model57,58. However, the mechanisms underlying the consequences of prenatal stress remain to be elucidated. This study investigated the effects of prenatal stress on depressive-like behavior and hippocampal neuroplasticity in male offspring. Studies have collectively shown that the rapid and significant effects of ketamine arise from its ability to rapidly reverse neuroplasticity deficits in animal models20,27–29,59. Ketamine acts by blocking the NMDA receptor and thus triggering a series of downstream effects that promote neuroplasticity and neurogenesis. The hippocampus is a key structure of the limbic system and can be damaged through stress-triggered hippocampal atrophy and impaired synaptic plasticity60. Neuronal morphology, such as dendrite complexity and spine density, are strongly correlated with neuronal function61.
In 2010, Li N et al.62 first found that after 24 h of reinjection, a single dose of ketamine (10 mg/kg) can rapidly reverse the behavioral and synaptic deficits caused by long-term chronic stress exposure in an mTOR-dependent manner. In recent years, an increasing number of researchers have focused on the antidepressant properties of ketamine and various studies have repeatedly confirmed that ketamine might serve to restore plasticity to induce its rapid antidepressant effect. The neurovascular plasticity of hippocampus is an important theory underlying major depression. Maryam Ardalan et al.63 confirmed that a single intraperitoneal injection of S-ketamine (15 mg/kg) can lead to an increase in synaptogenesis and vascularization within 24 h after a single injection in a genetic rat model of depression. Another of their study64 showed that a single intraperitoneal dose of S-ketamine (15 mg/kg) can affect the morphology of the hippocampal astrocytes and the serum level of BDNF within an hour. Unexceptionally, the study by Giulia Treccani et al.33 has demonstrated that a single dose of S-ketamine (15 mg/kg) reversed hippocampal dendritic spine deficits in flinders sensitive line rats within 1 h of administration. As indicated by the results of Golgi staining in our study, S-ketamine quickly reversed the reduction in complexity of pyramidal neurons in the hippocampal CA1 area of the PNS offspring, which is in agreement with reports in other depression models.
Prolonged stress and depression are known to cause atrophy of spine and synapses in brain regions implicated in depression, notably the prefrontal cortex and hippocampus. Dysregulation of synaptic formation and plasticity in the hippocampus have been implicated in depressed patients and rodent models of depression27,65. A reduction in the dendritic spine density usually reflects a reduction in the number of synapses and is intimately linked to synaptic transmission. Our results showed a rapid increase in the density of dendritic spines in the hippocampal CA1 area of PNS offspring 24 h after a single ketamine injection. Synaptic morphology and structural plasticity have a great effect on synapse function66. The postsynaptic density is a vital component of dendritic spines and is a protein density attached to the postsynaptic membrane, serving as a signaling apparatus. Furthermore, the thickness of the postsynaptic density is a marker of synaptic strength. The synaptic cleft is a space between presynaptic membrane and postsynaptic membrane is the key structure in transmitting action potentials and chemical transmitters. As the space grows larger, the signaling becomes slower. To obtain further evidence that the beneficial effect of S-ketamine on the altered behaviors of PNS offspring is associated with synaptic plasticity, we closely examined synaptic ultra-microstructure in CA1 using TEM. An in-depth analysis of the synaptic ultrastructure revealed that S-ketamine reversed the decreased thickness of the postsynaptic density in the hippocampal CA1 area of the PNS offspring. Neither prenatal stress nor S-ketamine administration showed significant changes in synaptic cleft width.
Previous studies indicated that BDNF, AKT/mTOR pathway and synaptic proteins, are crucial for the maintenance of the synaptic structure and function and correlated with symptoms of depression26,67. Chronic stress can enhance NMDAR and reduce AMPAR activities that decrease Akt signaling, which further regulates downstream effectors of inside the AKT/mTOR pathway68,69. NMDA-R inhibition increases glutamate release to stimulate AMPA glutamate receptors, which increase release of BDNF, enhances TrkB receptor stimulation, and activates Akt/mTORC1 signaling70. In the current study, a single dose of S-Ketamine increased BDNF release and stimulated AKT/mTOR signaling, which in turn increased levels of synaptic proteins (PSD95). However, S-ketamine administration did not return synaptophysin to normal levels, indicating that S-ketamine may rely more heavily on PSD95 to improve the synaptic plasticity than on synaptophysin.
There are several limitations in our study design that need to be resolved through further exploration. Firstly, this experiment was performed exclusively on male offspring. Secondly, because the mice died immediately after the behavioral tests, we don’t know how long S-ketamine’s antidepressant-like effects can last. Thirdly, only the hippocampus was examined in this study, thus future studies should explore other brain regions, especially the prefrontal cortex, to more fully assess the anti-depressive effects of S-ketamine. Furthermore, in future studies in PNS offspring, we would like to establish whether S-ketamine has advantages over other antidepressants.
In conclusion, the present study suggested that a single subanesthetic dose of S-ketamine had a beneficial effect on treatment of PNS-induced depression-like behaviors such as anhedonia and despair. In addition, hippocampal atrophy and reduced synaptic plasticity may be the root cause of the offspring’s depression. S-ketamine improved neuroplasticity by enhancing mTOR phosphorylation and promoting the release of BDNF, thus contributing to resistance to depression.
Summary
Collectively, the present study suggested that a single subanesthetic dose of S-ketamine had a beneficial effect on treatment of PNS-induced depression-like behaviors such as anhedonia and despair. In addition, hippocampal atrophy and reduced synaptic plasticity may be the root cause of the offspring’s depression. S-ketamine improved neuroplasticity by enhancing mTOR phosphorylation and promoting the release of BDNF, thus contributing to resistance to depression.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
Yan Zhang: Investigation, Writing – original draft, Writing – review & editing. Chuke Wei: Investigation, Methodology . Ping Wang: Investigation. Liucheng Zheng: Data curation. Yang Cheng: Data curation. Zhenhua Ren: Conceptualization. Yuhong Jin: Conceptualization. Yao Yuyou: Project administration, Funding acquisition. Huanzhong Liu: Review & editing, Funding acquisition. All authors reviewed the manuscript.
Funding
This study was supported by the National Clinical Key Specialty Project Foundation (CN) and the National Natural Science Foundation of China (Grant No. 81773452).
Data availability
The data that support the findings of the study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yu-You Yao, Email: yaoanqi71@aliyun.com.
Huan-Zhong Liu, Email: huanzhongliu@ahmu.edu.cn.
References
- 1.Phillips, D. I. & Jones, A. Fetal programming of autonomic and HPA function: do people who were small babies have enhanced stress responses? J. Physiol.572 (Pt 1), 45–50 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mbiydzenyuy, N. E., Hemmings, S. M. J. & Qulu, L. Prenatal maternal stress and offspring aggressive behavior: intergenerational and transgenerational inheritance. Front. Behav. Neurosci.16, 977416 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis, E. P. et al. Prenatal Maternal Stress, child cortical thickness, and adolescent depressive symptoms. Child. Dev.91 (2), e432–e450 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Lv, Y. et al. Role of corticotropin-releasing hormone in the impact of chronic stress during pregnancy on inducing depression in male offspring mice. Brain Res.1747, 147029 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Li, Y. et al. Paeoniflorin ameliorates depressive-like behavior in prenatally stressed offspring by restoring the HPA axis- and glucocorticoid receptor- associated dysfunction. J. Affect. Disord.274, 471–481 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Tirumalaraju, V. et al. Risk of Depression in the adolescent and adult offspring of mothers with Perinatal Depression: a systematic review and Meta-analysis. JAMA Netw. Open.3 (6), e208783 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fatima, M. et al. Effects of chronic unpredictable mild stress induced prenatal stress on neurodevelopment of neonates: role of GSK-3β. Sci. Rep.9 (1), 1305–1305 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li, Y. et al. Prenatal stress leads to the altered maturation of Corticostriatal synaptic plasticity and related behavioral impairments through epigenetic modifications of dopamine D2 receptor in mice. Mol. Neurobiol.58 (1), 317–328 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Majidi-Zolbanin, J. et al. Prenatal maternal immune activation increases anxiety- and depressive-like behaviors in offspring with experimental autoimmune encephalomyelitis. Neuroscience. 294, 69–81 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Depino, A. M. Early prenatal exposure to LPS results in anxiety- and depression-related behaviors in adulthood. Neuroscience. 299, 56–65 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Jianguo, L. et al. Altered gut metabolome contributes to depression-like behaviors in rats exposed to chronic unpredictable mild stress. Translational Psychiatry. 9 (1), 40–40 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu, H. et al. A 1H-NMR-Based metabonomic study on the anti-depressive effect of the total alkaloid of Corydalis Rhizoma. Molecules. 20 (6), 10047–10064 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diagnostic and statistical manual of mental disorders: DSM-5™, 5th ed. Diagnostic and statistical manual of mental disorders: DSM-5™, 5th ed. Arlington, VA, US: American Psychiatric Publishing, Inc. xliv, 947-xliv, 947. (2013).
- 14.Gomes, F. V., Zhu, X. & Grace, A. A. Stress during critical periods of development and risk for schizophrenia. Schizophr Res.213, 107–113 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson, R. M. et al. Maternal depression during pregnancy and the postnatal period: risks and possible mechanisms for offspring depression at age 18 years. JAMA Psychiatry. 70 (12), 1312–1319 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beversdorf, D. Q. et al. Timing of prenatal stressors and autism. J. Autism Dev. Disord. 35 (4), 471–478 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Whelan, Y. M. et al. Pathways from maternal depressive symptoms to adolescent depressive symptoms: the unique contribution of irritability symptoms. J. Child. Psychol. Psychiatry. 56 (10), 1092–1100 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pawlby, S. et al. Antenatal depression predicts depression in adolescent offspring: prospective longitudinal community-based study. J. Affect. Disord.113 (3), 236–243 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Plant, D. T. et al. Maternal depression during pregnancy and offspring depression in adulthood: role of child maltreatment. Br. J. Psychiatry. 207 (3), 213–220 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballard, E. D. & Zarate, C. A. Jr. The role of dissociation in ketamine’s antidepressant effects. Nat. Commun.11 (1), 6431–6431 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canuso, C. M. et al. Efficacy and Safety of Intranasal Esketamine for the Rapid reduction of symptoms of Depression and Suicidality in patients at imminent risk for suicide: results of a Double-Blind, randomized, placebo-controlled study. Am. J. Psychiatry. 175 (7), 620–630 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Daly, E. J. et al. Efficacy of Esketamine nasal spray plus oral antidepressant treatment for Relapse Prevention in patients with treatment-resistant depression: a Randomized Clinical Trial. JAMA Psychiatry. 76 (9), 893–903 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith-Apeldoorn, S. Y. et al. Maintenance ketamine treatment for depression: a systematic review of efficacy, safety, and tolerability. Lancet Psychiatry. 9 (11), 907–921 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Grunebaum, M. F. et al. Ketamine for Rapid reduction of suicidal thoughts in Major Depression: a midazolam-controlled Randomized Clinical Trial. Am. J. Psychiatry. 175 (4), 327–335 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Administration, U. F. D. FDA approves new nasal spray medication for treatment-resistant depression; available only at a certified doctor’s office or clinic. https://www.fda.gov/news-events/press-announcements/fda-approves-new-nasal-spray-medication-treatment-resistant-depression-available-only-certified
- 26.Duman, R. S. et al. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat. Med.22 (3), 238–249 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price, R. B. & Duman, R. Neuroplasticity in cognitive and psychological mechanisms of depression: an integrative model. Mol. Psychiatry. 25 (3), 530–543 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kadriu, B. et al. Ketamine and serotonergic psychedelics: common mechanisms underlying the effects of Rapid-Acting antidepressants. Int. J. Neuropsychopharmacol.24 (1), 8–21 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savalia, N. K., Shao, L. X. & Kwan, A. C. A dendrite-focused Framework for understanding the actions of ketamine and psychedelics. Trends Neurosci.44 (4), 260–275 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu, W. et al. Changes in hippocampal plasticity in Depression and therapeutic approaches influencing these changes. Neural Plast.2020, p8861903 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ribeiro, D. E. et al. P2X7 receptor signaling in stress and depression. Int. J. Mol. Sci. 2019, 20, 2778; doi:10.3390/ijms20112778 [DOI] [PMC free article] [PubMed]
- 32.Distler, U. et al. Proteomic analysis of Brain Region and Sex-Specific synaptic protein expression in the adult mouse brain. Cells 2020, 9, 313; doi:10.3390/cells9020313 [DOI] [PMC free article] [PubMed]
- 33.Treccani, G. et al. Reverses hippocampal dendritic spine deficits in Flinders Sensitive line rats within 1 h of Administration. Mol. Neurobiol.56 (11), 7368–7379 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Ren, Z. et al. Low-dose S-ketamine exerts antidepressant-like effects via enhanced hippocampal synaptic plasticity in postpartum depression rats. Neurobiol. Stress. 16, 100422–100422 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ardalan, M. et al. S-Ketamine rapidly reverses synaptic and vascular deficits of Hippocampus in Genetic Animal Model of Depression. Int. J. Neuropsychopharmacol.20 (3), 247–256 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Querfurth, H. & Lee, H. K. Mammalian/mechanistic target of rapamycin (mTOR) complexes in neurodegeneration. Mol. Neurodegeneration. 16 (1), 44–44 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ignácio, Z. M. et al. New perspectives on the involvement of mTOR in depression as well as in the action of antidepressant drugs. Br. J. Clin. Pharmacol.82 (5), 1280–1290 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin, J. E. et al. Soluble whey protein hydrolysate ameliorates muscle Atrophy Induced by Immobilization via regulating the PI3K/Akt pathway in C57BL/6 mice. Nutrients 2020, 12, 0; doi:10.3390/nu12110000 [DOI] [PMC free article] [PubMed]
- 39.Pazini, F. L. et al. mTORC1-dependent signaling pathway underlies the rapid effect of creatine and ketamine in the novelty-suppressed feeding test. Chem. Biol. Interact.332, 109281 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Modarresi, F. et al. Inhibition of natural antisense transcripts in vivo results in gene-specific transcriptional upregulation. Nat. Biotechnol.30 (5), 453–459 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sales, A. J. et al. Cannabidiol Induces Rapid and Sustained Antidepressant-Like effects through increased BDNF signaling and synaptogenesis in the Prefrontal Cortex. Mol. Neurobiol.56 (2), 1070–1081 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Yang, C. et al. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 5 (9), e632 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molosh, A. I. et al. Social learning and amygdala disruptions in Nf1 mice are rescued by blocking p21-activated kinase. Nat. Neurosci.17 (11), 1583–1590 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puighermanal, E. et al. Functional and molecular heterogeneity of D2R neurons along dorsal ventral axis in the striatum. Nat. Commun.11 (1), 1957–1957 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang, K. et al. Role of inflammatory bone markers in the antidepressant actions of (R)-Ketamine in a chronic social defeat stress model. Int. J. Neuropsychopharmacol.21 (11), 1025–1030 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fang, W. et al. Metformin ameliorates stress-induced depression-like behaviors via enhancing the expression of BDNF by activating AMPK/CREB-mediated histone acetylation. J. Affect. Disord.260, 302–313 (2020). [DOI] [PubMed] [Google Scholar]
- 47.Jing, G. et al. Erbin protects against sepsis-associated encephalopathy by attenuating microglia pyroptosis via IRE1α/Xbp1s-Ca(2+) axis. J. Neuroinflammation. 19 (1), 237 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi, H. et al. β-glucan attenuates cognitive impairment via the gut-brain axis in diet-induced obese mice. Microbiome. 8 (1), 143 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pazini, F. L. et al. Creatine, similar to ketamine, Counteracts Depressive-Like Behavior Induced by Corticosterone via PI3K/Akt/mTOR pathway. Mol. Neurobiol.53 (10), 6818–6834 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Collaborators, G. B. D. M. D. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of Disease Study 2019. The lancet. Psychiatry. 9 (2), 137–150 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng, Y. et al. Gestational stress induces depressive-like and anxiety-like phenotypes through epigenetic regulation of BDNF expression in offspring hippocampus. Epigenetics. 11 (2), 150–162 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Warner, V. et al. The course of major depression in the offspring of depressed parents. Incidence, recurrence, and recovery. Arch. Gen. Psychiatry. 49 (10), 795–801 (1992). [DOI] [PubMed] [Google Scholar]
- 53.Nagy, D. et al. Differential effects of an NR2B NAM and ketamine on synaptic potentiation and Gamma Synchrony: relevance to Rapid-Onset antidepressant efficacy. Neuropsychopharmacology. 41 (6), 1486–1494 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li, N. et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 329 (5994), 959–964 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu, R. et al. Transgenerational impairment of hippocampal Akt-mTOR signaling and behavioral deficits in the offspring of mice that experience postpartum depression-like illness. Prog Neuropsychopharmacol. Biol. Psychiatry. 73, 11–18 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Maneenet, J. et al. Kleeb Bua Daeng, a Thai Traditional Herbal Formula, ameliorated unpredictable chronic mild stress-Induced Cognitive impairment in ICR mice. Molecules 2019, 24, 4587; doi:10.3390/molecules24244587 [DOI] [PMC free article] [PubMed]
- 57.Duman, R. S. & Aghajanian, G. K. Synaptic Dysfunction in Depression: Potential Therapeutic Targets338p. 68–72 (Science, 2012). 6103. [DOI] [PMC free article] [PubMed]
- 58.Lin, L. Y. et al. Early-life stress leads to impaired spatial learning and memory in middle-aged ApoE4-TR mice. Mol. Neurodegener. 11 (1), 51 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Höflich, A. et al. Translating the immediate effects of S-Ketamine using hippocampal subfield analysis in healthy subjects-results of a randomized controlled trial. Translational Psychiatry. 11 (1), 200–200 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang, E. et al. Adult Neurogenesis and Psychiatric disorders. Cold Spring Harb Perspect Biol 2015;8:a019026 [DOI] [PMC free article] [PubMed]
- 65.Hladik, D. et al. Combined treatment with low-dose Ionizing Radiation and ketamine induces adverse changes in CA1 neuronal structure in male Murine Hippocampi. Int. J. Mol. Sci. 2019, 20, 6103; doi:10.3390/ijms20236103 [DOI] [PMC free article] [PubMed]
- 62.Li, N. et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol. Psychiatry. 69 (8), 754–761 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ardalan, M. et al. Rapidly reverses synaptic and vascular deficits of Hippocampus in Genetic Animal Model of Depression. Int. J. Neuropsychopharmacol.20 (3), 247–256 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ardalan, M. et al. Rapid effects of S-ketamine on the morphology of hippocampal astrocytes and BDNF serum levels in a sex-dependent manner. Eur. Neuropsychopharmacol.32, 94–103 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aleksandrova, L. R., Wang, Y. T. & Phillips, A. G. Evaluation of the Wistar-Kyoto rat model of depression and the role of synaptic plasticity in depression and antidepressant response. Neurosci. Biobehav Rev.105, 1–23 (2019). [DOI] [PubMed] [Google Scholar]
- 66.Ran, Y. H. et al. YL-0919, a dual 5-HT(1A) partial agonist and SSRI, produces antidepressant- and anxiolytic-like effects in rats subjected to chronic unpredictable stress. Acta Pharmacol. Sin. 39 (1), 12–23 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duman, R. S. & Voleti, B. Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci.35 (1), 47–56 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou, J. J. et al. Enhanced hypothalamic NMDA receptor activity contributes to hyperactivity of HPA Axis in chronic stress in male rats. Endocrinology. 159 (3), 1537–1546 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang, J. et al. Involvement of normalized NMDA receptor and mTOR-related signaling in rapid antidepressant effects of Yueju and ketamine on chronically stressed mice. Sci. Rep.5, 13573 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krystal, J. H., Charney, D. S. & Duman, R. S. A New Rapid-acting antidepressant. Cell. 181 (1), 7 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of the study are available from the corresponding author upon reasonable request.