Abstract
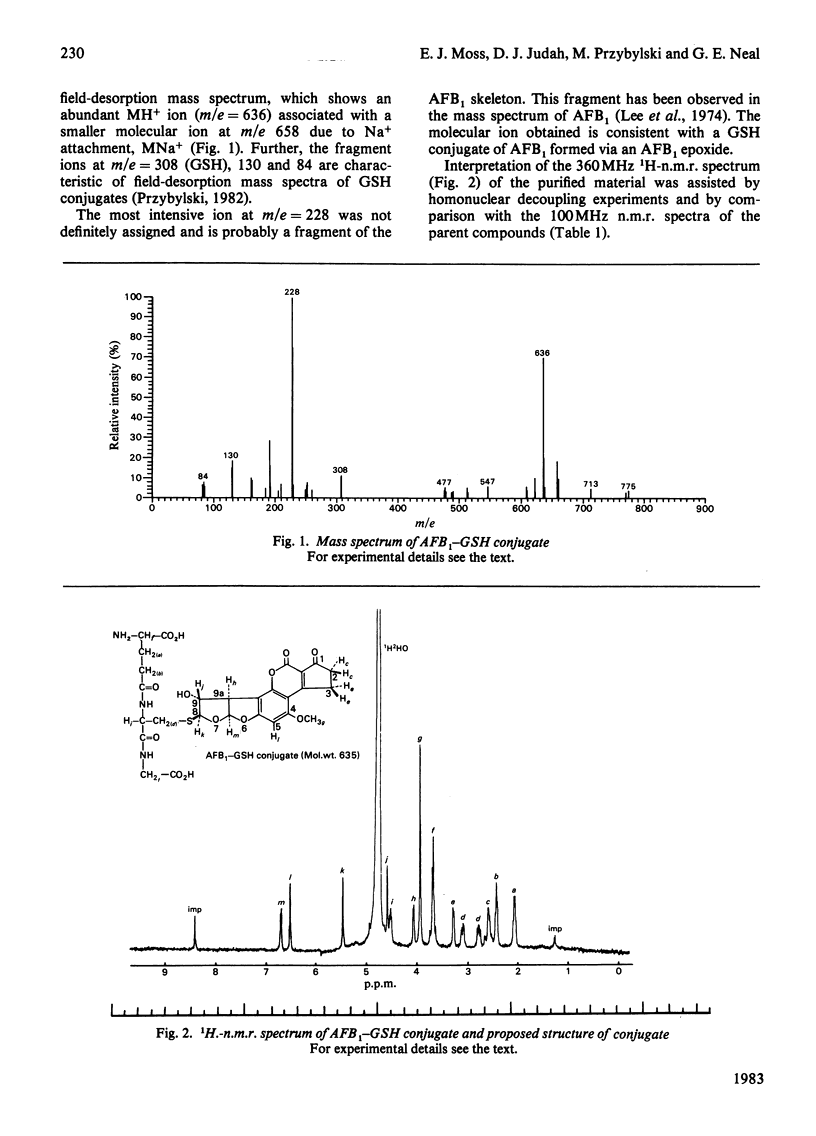
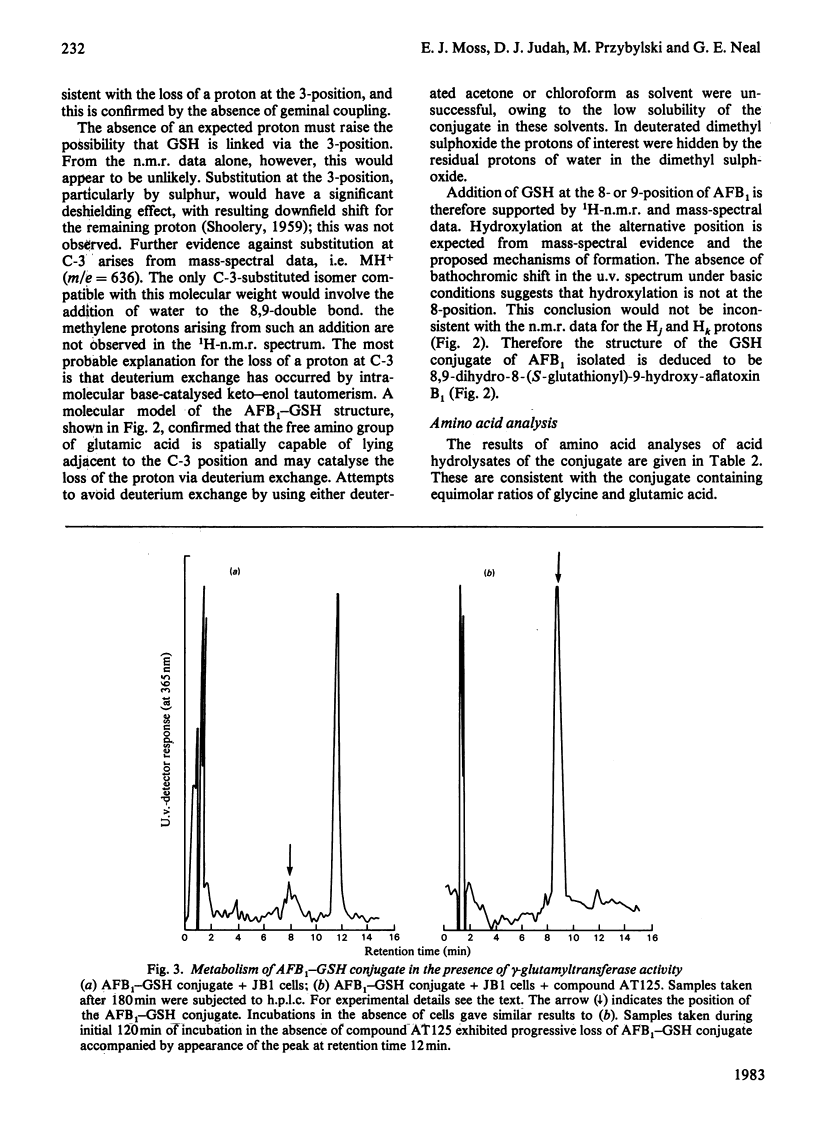
A system for the formation of an aflatoxin B1-reduced glutathione conjugate in vitro was developed, capable of yielding 80% conversion of aflatoxin B1 into the conjugate. A reverse-phase high-pressure-liquid-chromatography system was also devised that not only facilitates improved resolution of the compound but that, by manipulation of the pH, is also capable of an extensive purification of the compound from other aflatoxin B1 metabolites in a single step. Material produced by these techniques, after further purification, has been used in 1H-n.m.r. and mass-spectroscopic studies. Results were obtained that support the proposed linkage of the aflatoxin B1 to reduced glutathione in a 1:1 molar ratio via a thioether linkage. Amino acid analyses were also consistent with this structure. The absence of a Schiff-base linkage of aflatoxin B1 8,9-dihydrodiol to glutamate was further demonstrated by the presence of a gamma-glutamyltransferase-catalysed-transferable glutamate moiety. These data are consistent with the structure 8,9-dihydro-8-(S-glutathionyl)-9-hydroxy-aflatoxin B1.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Büchi G., Foulkes D. M., Kurono M., Mitchell G. F., Schneider R. S. The total synthesis of racemic aflatoxin B1. J Am Chem Soc. 1967 Dec 6;89(25):6745–6753. doi: 10.1021/ja01001a062. [DOI] [PubMed] [Google Scholar]
- Campbell T. C., Hayes J. R., Newberne P. M. Dietary lipotropes, hepatic microsomal mixed-function oxidase activities, and in vivo covalent binding of aflatoxin B1 in rats. Cancer Res. 1978 Dec;38(12):4569–4573. [PubMed] [Google Scholar]
- Campbell T. C., Hayes J. R. The role of aflatoxin metabolism in its toxic lesion. Toxicol Appl Pharmacol. 1976 Feb;35(2):199–222. doi: 10.1016/0041-008x(76)90282-9. [DOI] [PubMed] [Google Scholar]
- Climie I. J., Hutson D. H., Morrison B. J., Stoydin G. Glutathione conjugation in the detoxication of (Z)-1,3-dichloropropene (a component of the nematocide D-D) in the rat. Xenobiotica. 1979 Mar;9(3):149–156. doi: 10.3109/00498257909038715. [DOI] [PubMed] [Google Scholar]
- Degen G. H., Neumann H. G. Differences in aflatoxin B1-susceptibility of rat and mouse are correlated with the capability in vitro to inactivate aflatoxin B1-epoxide. Carcinogenesis. 1981;2(4):299–306. doi: 10.1093/carcin/2.4.299. [DOI] [PubMed] [Google Scholar]
- Degen G. H., Neumann H. G. The major metabolite of aflatoxin B1 in the rat is a glutathione conjugate. Chem Biol Interact. 1978 Sep;22(2-3):239–255. doi: 10.1016/0009-2797(78)90129-1. [DOI] [PubMed] [Google Scholar]
- Ding J. L., Smith G. D., Peters T. J. Subcellular localization and isolation of gamma-glutamyltransferase from rat hepatoma cells. Biochim Biophys Acta. 1981 Oct 13;661(2):191–198. doi: 10.1016/0005-2744(81)90003-6. [DOI] [PubMed] [Google Scholar]
- Essigmann J. M., Croy R. G., Nadzan A. M., Busby W. F., Jr, Reinhold V. N., Büchi G., Wogan G. N. Structural identification of the major DNA adduct formed by aflatoxin B1 in vitro. Proc Natl Acad Sci U S A. 1977 May;74(5):1870–1874. doi: 10.1073/pnas.74.5.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner R. C., Martin C. N., Smith J. R., Coles B. F., Tolson M. R. Comparison of aflatoxin B1 and aflatoxin G1 binding to cellular macromolecules in vitro, in vivo and after peracid oxidation; characterisation of the major nucleic acid adducts. Chem Biol Interact. 1979 Jun;26(1):57–73. doi: 10.1016/0009-2797(79)90093-0. [DOI] [PubMed] [Google Scholar]
- Garner R. C., Miller E. C., Miller J. A. Liver microsomal metabolism of aflatoxin B 1 to a reactive derivative toxic to Salmonella typhimurium TA 1530. Cancer Res. 1972 Oct;32(10):2058–2066. [PubMed] [Google Scholar]
- Irving C. C., Wiseman R., Jr, Hill J. T. Biliary excretion of the O-glucuronide of N-hydroxy-2-acetylamino-fluorene by the rat and rabbit. Cancer Res. 1967 Dec;27(12):2309–2317. [PubMed] [Google Scholar]
- Lee L. S., Stanley J. B., Cucullu A. F., Pons W. A., Jr, Goldblatt L. A. Ammoniation of aflatoxin B1: isolation and identification of the major reaction product. J Assoc Off Anal Chem. 1974 May;57(3):626–631. [PubMed] [Google Scholar]
- Lotlikar P. D., Insetta S. M., Lyons P. R., Jhee E. C. Inhibition of microsome-mediated binding of aflatoxin B1 to DNA by glutathione S-transferase. Cancer Lett. 1980 Apr;9(2):143–149. doi: 10.1016/0304-3835(80)90118-4. [DOI] [PubMed] [Google Scholar]
- Manson M. M., Green J. A. Effect of microsomally activated AFB1 on GGT activity in 3 rat liver cell lines. Br J Cancer. 1982 Jun;45(6):945–952. doi: 10.1038/bjc.1982.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson M. M., Legg R. F., Watson J. V., Green J. A., Neal G. E. An examination of the relative resistances to aflatoxin B1 and susceptibilities to gamma-glutamyl p-phenylene diamine mustard of gamma-glutamyl transferase negative and positive cell lines. Carcinogenesis. 1981;2(7):661–670. doi: 10.1093/carcin/2.7.661. [DOI] [PubMed] [Google Scholar]
- Metcalfe S. A., Colley P. J., Neal G. E. A comparison of the effects of pretreatment with phenobarbitone and 3-methylcholanthrene on the metabolism of aflatoxin B1 by rat liver microsomes and isolated hepatocytes in vitro. Chem Biol Interact. 1981 May;35(2):145–157. doi: 10.1016/0009-2797(81)90139-3. [DOI] [PubMed] [Google Scholar]
- Mulder G. J., Unruh L. E., Evans F. E., Ketterer B., Kadlubar F. F. Formation and identification of glutathione conjugates from 2-nitrosofluorene and N-hydroxy-2-aminofluorene. Chem Biol Interact. 1982 Mar 1;39(1):111–127. doi: 10.1016/0009-2797(82)90010-2. [DOI] [PubMed] [Google Scholar]
- Neal G. E., Colley P. J. Some high-performance liquid-chromatographic studies of the metabolism of aflatoxins by rat liver microsomal preparations. Biochem J. 1978 Sep 15;174(3):839–851. doi: 10.1042/bj1740839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal G. E., Colley P. J. The formation of 2,3-dihydro-2,3-dihydroxy aflatoxin B1 by the metabolism of aflatoxin B1 in vitro by rat liver microsomes. FEBS Lett. 1979 May 15;101(2):382–386. doi: 10.1016/0014-5793(79)81049-2. [DOI] [PubMed] [Google Scholar]
- Neal G. E., Judah D. J., Stirpe F., Patterson D. S. The formation of 2,3-dihydroxy-2,3-dihydro-aflatoxin B1 by the metabolism of aflatoxin B1 by liver microsomes isolated from certain avian and mammalian species and the possible role of this metabolite in the acute toxicity of aflatoxin B1. Toxicol Appl Pharmacol. 1981 May;58(3):431–437. doi: 10.1016/0041-008x(81)90095-8. [DOI] [PubMed] [Google Scholar]
- Neal G. E., Mattocks A. R., Judah D. J. The microsomal activation of aflatoxin B1 and 2-(N-ethylcarbamoyloxymethyl)furan in vitro using a novel diffusion apparatus. Biochim Biophys Acta. 1979 Jun 1;585(1):134–142. doi: 10.1016/0304-4165(79)90333-7. [DOI] [PubMed] [Google Scholar]
- Neal G. E., Metcalfe S. A., Legg R. F., Judah D. H., Green J. A. Mechanism of the resistance to cytotoxicity which precedes aflatoxin B1 hepatocarcinogenesis. Carcinogenesis. 1981;2(5):457–461. doi: 10.1093/carcin/2.5.457. [DOI] [PubMed] [Google Scholar]
- Przybylski M. Identification of metabolism pathways of anticancer drugs by high-pressure liquid chromatography in combination with field desorption mass spectrometry. Arzneimittelforschung. 1982;32(9):995–1012. [PubMed] [Google Scholar]
- Przybylski M., Preiss J., Dennebaum R., Fischer J. Identification and quantitation of methotrexate and methotrexate metabolites in clinical high-dose therapy by high pressure liquid chromatography and field desorption mass spectrometry. Biomed Mass Spectrom. 1982 Jan;9(1):22–32. doi: 10.1002/bms.1200090106. [DOI] [PubMed] [Google Scholar]
- Reed D. J., Ellis W. W., Meck R. A. The inhibition of gamma-glutamyl transpeptidase and glutathione metabolism of isolated rat kidney cells by L-(alpha S, 5S)-alpha-amino-3-chloro-4, 5-dihydro-5-isoxazoleacetic acid (AT-125; NSC-163501). Biochem Biophys Res Commun. 1980 Jun 30;94(4):1273–1277. doi: 10.1016/0006-291x(80)90557-4. [DOI] [PubMed] [Google Scholar]
- Swenson D. H., Lin J. K., Miller E. C., Miller J. A. Aflatoxin B1-2,3-oxide as a probable intermediate in the covalent binding of aflatoxins B1 and B2 to rat liver DNA and ribosomal RNA in vivo. Cancer Res. 1977 Jan;37(1):172–181. [PubMed] [Google Scholar]
- Ueno I., Friedman L., Stone C. L. Species difference in the binding of aflatoxin B1 to hepatic macromolecules. Toxicol Appl Pharmacol. 1980 Jan;52(1):177–180. doi: 10.1016/0041-008x(80)90257-4. [DOI] [PubMed] [Google Scholar]


