Abstract
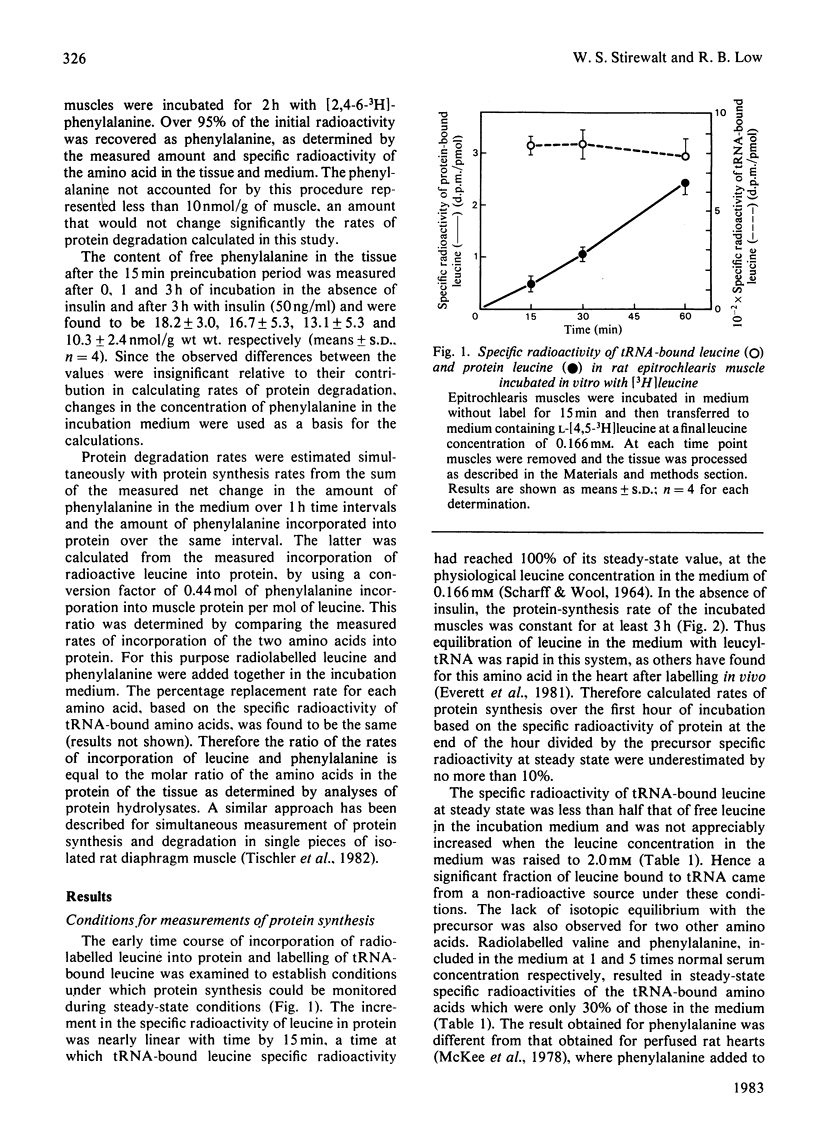
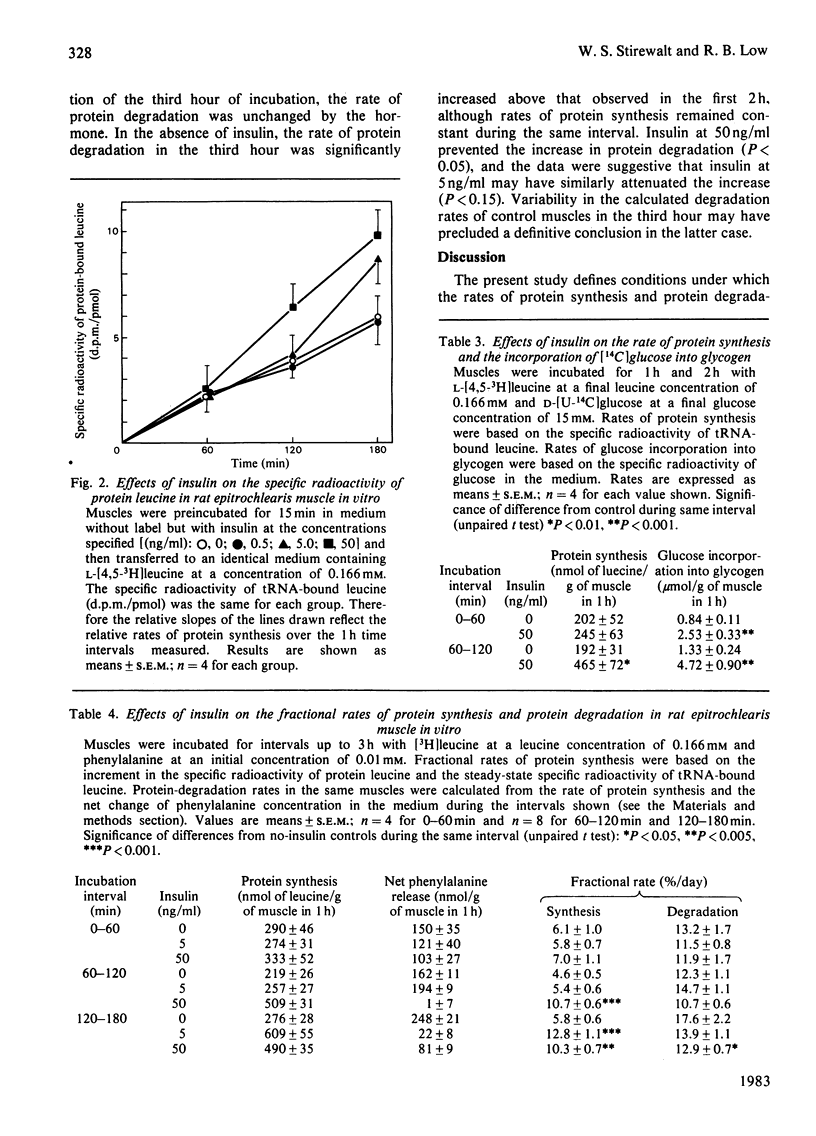
Rates of protein synthesis and degradation were measured in the isolated rat epitrochlearis muscle by radiotracer techniques, by using the specific radioactivity of tRNA-bound amino acid as precursor for protein synthesis. The tissue maintained linear rates of protein synthesis for 3 h of incubation in the presence of amino acids and glucose and in the absence of insulin. Under these conditions, however, the muscles were in negative nitrogen balance, with rates of protein degradation exceeding rates of protein synthesis. Under steady-state conditions of labelling, the specific radioactivities of tRNA-bound leucine, phenylalanine and valine were significantly less than their respective values in the incubation medium, at concentrations in the medium varying from 1 to 10 times those in normal rat serum. Insulin caused a dose- and time-dependent increase in tRNA-based protein synthesis rates, more than doubling rates at 5 and 50 ng of insulin/ml. At the lower, physiological, concentration of insulin, the stimulation of protein synthesis was not observed until the third hour of incubation with the hormone, whereas the rate of protein synthesis at the higher concentration was elevated during the second hour. There were no delays in the stimulation by insulin of glucose conversion into glycogen. The delayed stimulatory effects of insulin on the rate of protein synthesis brought the tissue to a nitrogen balance near zero. The presence of the hormone also prevented the increase in the rate of protein degradation seen in the third hour of incubation in the absence of the hormone. These studies demonstrate the viability of the incubated rat epitrochlearis muscle with respect to protein metabolism and sensitivity to the protein anabolic effects of physiological concentrations of insulin, and indicate that the preparation is a suitable experimental model for the study of the control of protein metabolism in fast-twitch skeletal muscle.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Airhart J., Kelley J., Brayden J. E., Low R. B., Stirewalt W. S. An ultramicro method of amino acid analysis: application to studies of protein metabolism in cultured cells. Anal Biochem. 1979 Jul 1;96(1):45–55. doi: 10.1016/0003-2697(79)90552-9. [DOI] [PubMed] [Google Scholar]
- Everett A. W., Prior G., Zak R. Equilibration of leucine between the plasma compartment and leucyl-tRNA in the heart, and turnover of cardiac myosin heavy chain. Biochem J. 1981 Jan 15;194(1):365–368. doi: 10.1042/bj1940365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaim K. E., Copenhaver M. E., Jefferson L. S. Effects of diabetes on protein synthesis in fast- and slow-twitch rat skeletal muscle. Am J Physiol. 1980 Jul;239(1):E88–E95. doi: 10.1152/ajpendo.1980.239.1.E88. [DOI] [PubMed] [Google Scholar]
- Frayn K. N., Maycock P. F. Regulation of protein metabolism by a physiological concentration of insulin in mouse soleus and extensor digitorum longus muscles. Effects of starvation and scald injury. Biochem J. 1979 Nov 15;184(2):323–330. doi: 10.1042/bj1840323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulks R. M., Li J. B., Goldberg A. L. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975 Jan 10;250(1):290–298. [PubMed] [Google Scholar]
- Garber A. J., Karl I. E., Kipnis D. M. Alanine and glutamine synthesis and release from skeletal muscle. I. Glycolysis and amino acid release. J Biol Chem. 1976 Feb 10;251(3):826–835. [PubMed] [Google Scholar]
- Goldberg A. L. Mechanisms of growth and atrophy of skeletal muscle. Muscle Biol. 1972;1:89–118. [PubMed] [Google Scholar]
- Goldspink D. F. The influence of immobilization and stretch on protein turnover of rat skeletal muscle. J Physiol. 1977 Jan;264(1):267–282. doi: 10.1113/jphysiol.1977.sp011667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink D. F. The influence of passive stretch on the growth and protein turnover of the denervated extensor digitorum longus muscle. Biochem J. 1978 Aug 15;174(2):595–602. doi: 10.1042/bj1740595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter H. R., Karl I. E., Klahr S., Kipnis D. M. Effects of reduced renal mass and dietary protein intake on amino acid release and glucose uptake by rat muscle in vitro. J Clin Invest. 1979 Aug;64(2):513–523. doi: 10.1172/JCI109489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson L. S., Koehler J. O., Morgan H. E. Effect of insulin on protein synthesis in skeletal muscle of an isolated perfused preparation of rat hemicorpus. Proc Natl Acad Sci U S A. 1972 Apr;69(4):816–820. doi: 10.1073/pnas.69.4.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson L. S., Li J. B., Rannels S. R. Regulation by insulin of amino acid release and protein turnover in the perfused rat hemicorpus. J Biol Chem. 1977 Feb 25;252(4):1476–1483. [PubMed] [Google Scholar]
- Jefferson L. S. Lilly Lecture 1979: role of insulin in the regulation of protein synthesis. Diabetes. 1980 Jun;29(6):487–496. doi: 10.2337/diab.29.6.487. [DOI] [PubMed] [Google Scholar]
- Jefferson L. S., Rannels D. E., Munger B. L., Morgan H. E. Insulin in the regulation of protein turnover in heart and skeletal muscle. Fed Proc. 1974 Apr;33(4):1098–1104. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Li J. B., Higgins J. E., Jefferson L. S. Changes in protein turnover in skeletal muscle in response to fasting. Am J Physiol. 1979 Mar;236(3):E222–E228. doi: 10.1152/ajpendo.1979.236.3.E222. [DOI] [PubMed] [Google Scholar]
- McKee E. E., Cheung J. Y., Rannels D. E., Morgan H. E. Measurement of the rate of protein synthesis and compartmentation of heart phenylalanine. J Biol Chem. 1978 Feb 25;253(4):1030–1040. [PubMed] [Google Scholar]
- Nesher R., Karl I. E., Kaiser K. E., Kipnis D. M. Epitrochlearis muscle. I. Mechanical performance, energetics, and fiber composition. Am J Physiol. 1980 Dec;239(6):E454–E460. doi: 10.1152/ajpendo.1980.239.6.E454. [DOI] [PubMed] [Google Scholar]
- Odedra B. R., Dalal S. S., Millward D. J. Muscle protein synthesis in the streptozotocin-diabetic rat. A possible role for corticosterone in the insensitivity to insulin infusion in vivo. Biochem J. 1982 Feb 15;202(2):363–368. doi: 10.1042/bj2020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odessey R., Parr B. Effect of insulin and leucine on protein turnover in rat soleus muscle after burn injury. Metabolism. 1982 Jan;31(1):82–87. [PubMed] [Google Scholar]
- Pain V. M., Garlick P. J. Effect of streptozotocin diabetes and insulin treatment on the rate of protein synthesis in tissues of the rat in vivo. J Biol Chem. 1974 Jul 25;249(14):4510–4514. [PubMed] [Google Scholar]
- Reeds P. J., Palmer R. M., Smith R. H. Protein and collagen synthesis in rat diaphragm muscle incubated in vitro: the effect of alterations in tension produced by electrical or mechanical means. Int J Biochem. 1980;11(1):7–14. doi: 10.1016/0020-711x(80)90274-8. [DOI] [PubMed] [Google Scholar]
- SCHARFF R., WOOL I. G. CONCENTRATION OF AMINO ACIDS IN RAT MUSCLE AND PLASMA. Nature. 1964 May 9;202:603–604. doi: 10.1038/202603a0. [DOI] [PubMed] [Google Scholar]
- Stirewalt W. S., Wool I. G., Cavicchi P. The relation of RNA and protein synthesis to the sedimentation of muscle ribosomes: effect of diabetes and insulin. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1885–1892. doi: 10.1073/pnas.57.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischler M. E., Desautels M., Goldberg A. L. Does leucine, leucyl-tRNA, or some metabolite of leucine regulate protein synthesis and degradation in skeletal and cardiac muscle? J Biol Chem. 1982 Feb 25;257(4):1613–1621. [PubMed] [Google Scholar]
- VON EHRENSTEIN G., LIPMANN F. Experiments on hemoglobin biosynthesis. Proc Natl Acad Sci U S A. 1961 Jul 15;47:941–950. doi: 10.1073/pnas.47.7.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak R., Martin A. F., Blough R. Assessment of protein turnover by use of radioisotopic tracers. Physiol Rev. 1979 Apr;59(2):407–447. doi: 10.1152/physrev.1979.59.2.407. [DOI] [PubMed] [Google Scholar]


