Abstract
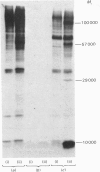
1. Incubation of a rat islet cell tumour homogenate with [gamma-32P]ATP resulted in the Ca2+-dependent phosphorylation of 100000-, 57000-, 29000-, 26000- and 14000-Mr proteins. The Ca2+ concentration required was in the low-microM range. 2. Isolated insulin granules did not exhibit Ca2+-dependent protein phosphorylation, whereas a soluble protein fraction showed the Ca2+-dependent phosphorylation of a 57000-Mr protein. Combination of insulin granules with the soluble protein fraction resulted in the additional Ca2+-dependent phosphorylations of 100000-, 29000- and 10000-Mr proteins. The latter phosphorylations were not enhanced by exogenous calmodulin, but nevertheless were inhibited by trifluoperazine. Removal of endogenous calmodulin from the soluble protein fraction before incubation with insulin granules did not abolish the Ca2+-dependent phosphorylations of the 100000-, 29000- and 10000-Mr proteins but rendered the Ca2+-dependent phosphorylation of the 57000-Mr soluble protein dependent on exogenous calmodulin. 3. The components of the soluble protein fraction responsible for the interaction with insulin granules bound to intact granules in a Ca2+-dependent manner. 4. After phosphorylation, the 29000-Mr protein remained attached to granules, whereas the 100000- and 10000-Mr proteins dissociated from granules. 5. These Ca2+-dependent phenomena may be of regulatory importance in the secretory mechanism.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amy C. M., Kirshner N. Phosphorylation of adrenal medulla cell proteins in conjunction with stimulation of catecholamine secretion. J Neurochem. 1981 Mar;36(3):847–854. doi: 10.1111/j.1471-4159.1981.tb01671.x. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Geisow M. J. Specific binding of 125I-calmodulin to and protein phosphorylation in adrenal chromaffin granule membranes. FEBS Lett. 1981 Aug 17;131(1):127–131. doi: 10.1016/0014-5793(81)80903-9. [DOI] [PubMed] [Google Scholar]
- Chick W. L., Warren S., Chute R. N., Like A. A., Lauris V., Kitchen K. C. A transplantable insulinoma in the rat. Proc Natl Acad Sci U S A. 1977 Feb;74(2):628–632. doi: 10.1073/pnas.74.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P., Burchell A., Foulkes J. G., Cohen P. T., Vanaman T. C., Nairn C. Identification of the Ca2+-dependent modulator protein as the fourth subunit of rabbit skeletal muscle phosphorylase kinase. FEBS Lett. 1978 Aug 15;92(2):287–293. doi: 10.1016/0014-5793(78)80772-8. [DOI] [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature. 1982 Apr 15;296(5858):613–620. doi: 10.1038/296613a0. [DOI] [PubMed] [Google Scholar]
- Harrison D. E., Ashcroft S. J. Effects of Ca2+, calmodulin and cyclic AMP on the phosphorylation of endogenous proteins by homogenates of rt islets of langerhans. Biochim Biophys Acta. 1982 Feb 2;714(2):313–319. doi: 10.1016/0304-4165(82)90339-7. [DOI] [PubMed] [Google Scholar]
- Hartshorne D. J., Siemankowski R. F. Regulation of smooth muscle actomyosin. Annu Rev Physiol. 1981;43:519–530. doi: 10.1146/annurev.ph.43.030181.002511. [DOI] [PubMed] [Google Scholar]
- Hutton J. C., Penn E. J., Jackson P., Hales C. N. Isolation and characterization of calmodulin from an insulin-secreting tumour. Biochem J. 1981 Mar 1;193(3):875–885. doi: 10.1042/bj1930875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton J. C., Penn E. J., Peshavaria M. Isolation and characterisation of insulin secretory granules from a rat islet cell tumour. Diabetologia. 1982 Oct;23(4):365–373. doi: 10.1007/BF00253746. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landt M., McDaniel M. L., Bry C. G., Kotagal N., Colca J. R., Lacy P. E., McDonald J. M. Calmodulin-activated protein kinase activity in rat pancreatic islet cell membranes. Arch Biochem Biophys. 1982 Jan;213(1):148–154. doi: 10.1016/0003-9861(82)90449-0. [DOI] [PubMed] [Google Scholar]
- Le Peuch C. J., Haiech J., Demaille J. G. Concerted regulation of cardiac sarcoplasmic reticulum calcium transport by cyclic adenosine monophosphate dependent and calcium--calmodulin-dependent phosphorylations. Biochemistry. 1979 Nov 13;18(23):5150–5157. doi: 10.1021/bi00590a019. [DOI] [PubMed] [Google Scholar]
- Nishikawa M., Tanaka T., Hidaka H. Ca2+-calmodulin-dependent phosphorylation and platelet secretion. Nature. 1980 Oct 30;287(5785):863–865. doi: 10.1038/287863a0. [DOI] [PubMed] [Google Scholar]
- Schatzman R. C., Wise B. C., Kuo J. F. Phospholipid-sensitive calcium-dependent protein kinase: inhibition by antipsychotic drugs. Biochem Biophys Res Commun. 1981 Feb 12;98(3):669–676. doi: 10.1016/0006-291x(81)91166-9. [DOI] [PubMed] [Google Scholar]
- Schubart U. K., Erlichman J., Fleischer N. The role of calmodulin in the regulation of protein phosphorylation and insulin release in hamster insulinoma cells. J Biol Chem. 1980 May 10;255(9):4120–4124. [PubMed] [Google Scholar]
- Schubart U. K., Fleischer N., Erlichman J. Ca2+-dependent protein phosphorylation and insulin release in intact hamster insulinoma cells. Inhibition by trifluoperazine. J Biol Chem. 1980 Dec 10;255(23):11063–11066. [PubMed] [Google Scholar]
- Sieghart W., Theoharides T. C., Alper S. L., Douglas W. W., Greengard P. Calcium-dependent protein phosphorylation during secretion by exocytosis in the mast cell. Nature. 1978 Sep 28;275(5678):329–331. doi: 10.1038/275329a0. [DOI] [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. Concentration of MgATP2- and other ions in solution. Calculation of the true concentrations of species present in mixtures of associating ions. Biochem J. 1976 Oct 1;159(1):1–5. doi: 10.1042/bj1590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Oka H., Yasuda H., Ikeda M., Cheng P. Y., Oda T. Effect of glucose on protein phosphorylation in rat pancreatic islets. Biochem Biophys Res Commun. 1981 Apr 15;99(3):987–993. doi: 10.1016/0006-291x(81)91259-6. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Perkins J. P., Brosom C. O., Ho E. S., Kreb E. G. Catlysis of the phosphrylaseinase actition reaction. J Biol Chem. 1971 Apr 10;246(7):1968–1976. [PubMed] [Google Scholar]
- Wollheim C. B., Sharp G. W. Regulation of insulin release by calcium. Physiol Rev. 1981 Oct;61(4):914–973. doi: 10.1152/physrev.1981.61.4.914. [DOI] [PubMed] [Google Scholar]