Abstract
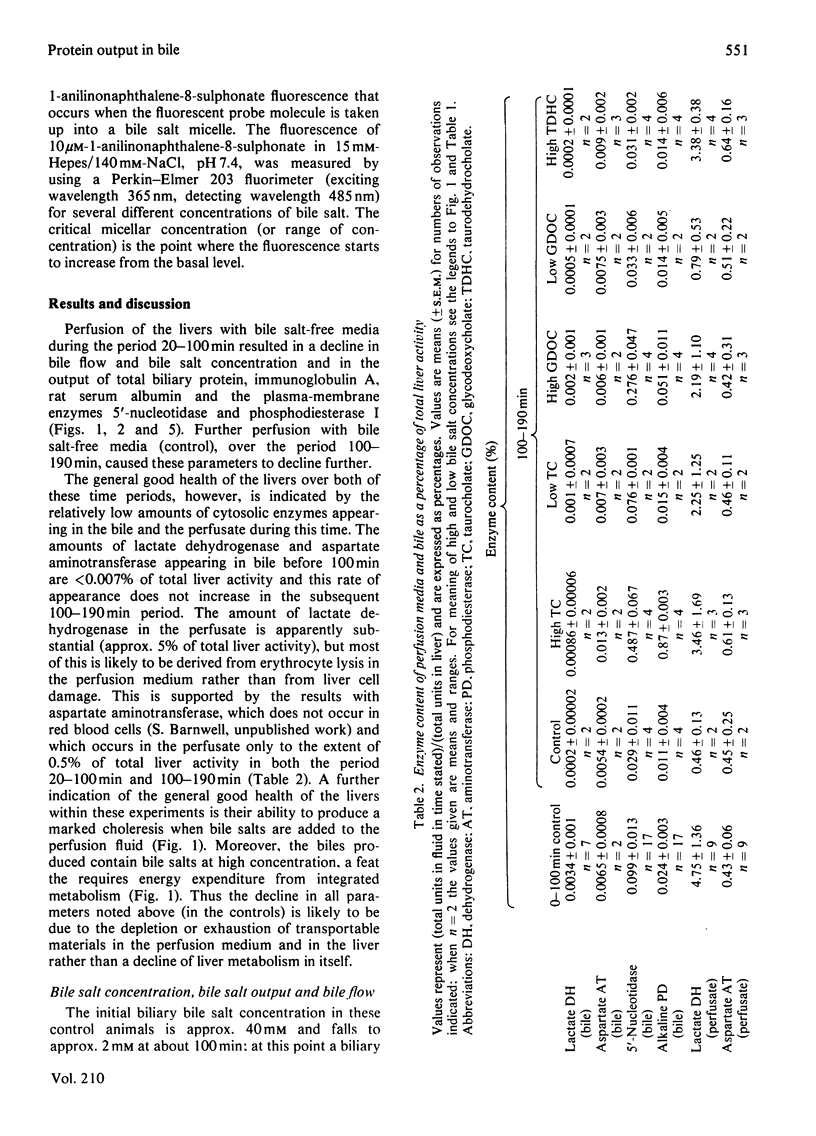
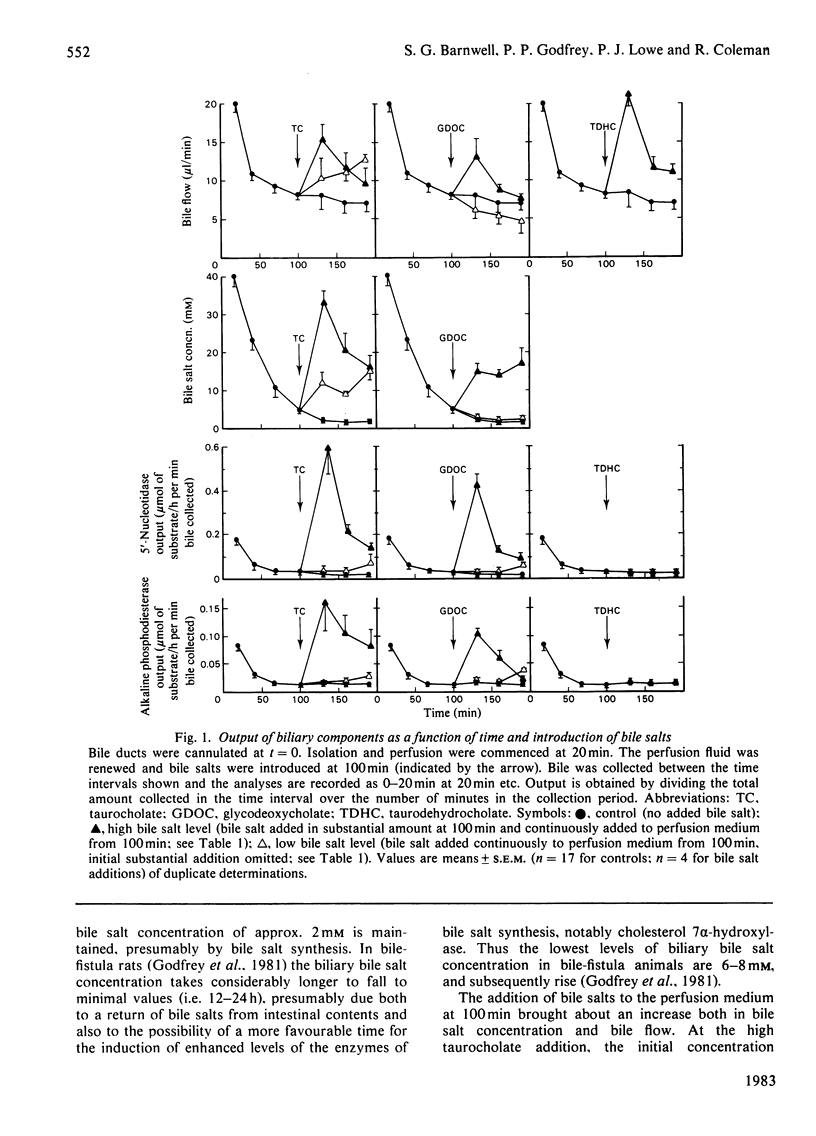
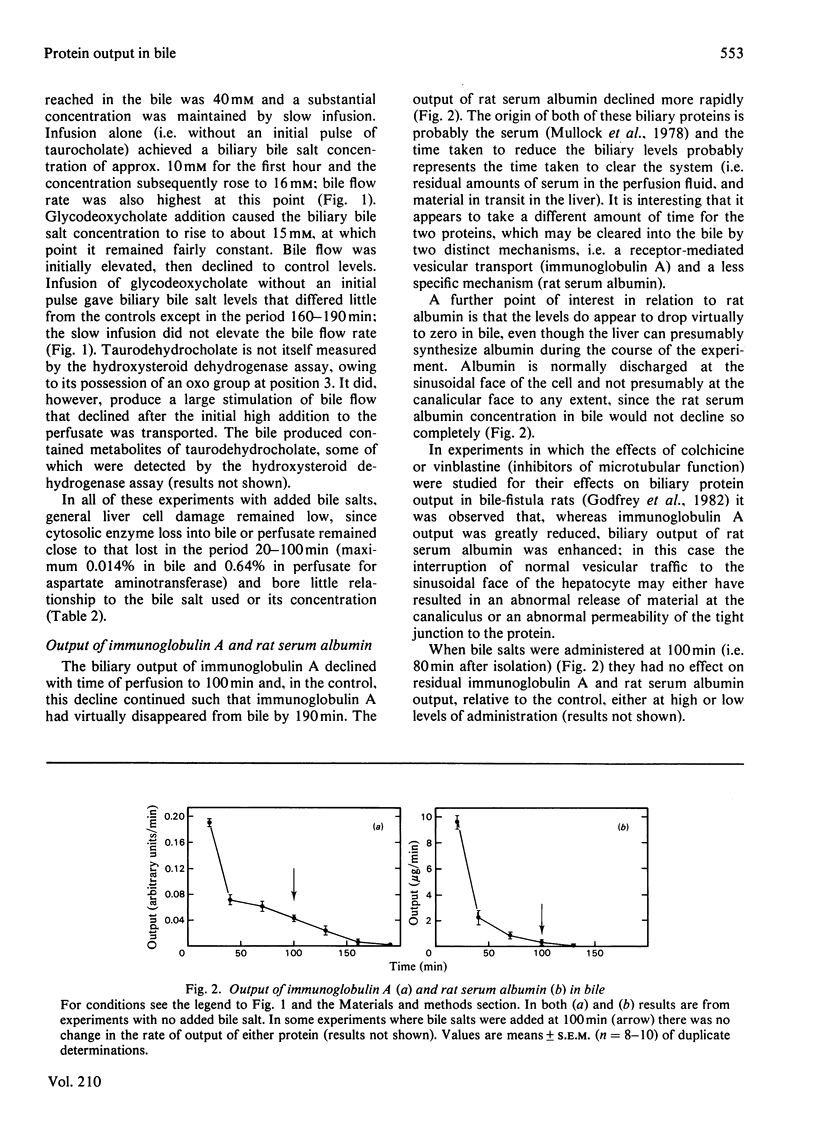
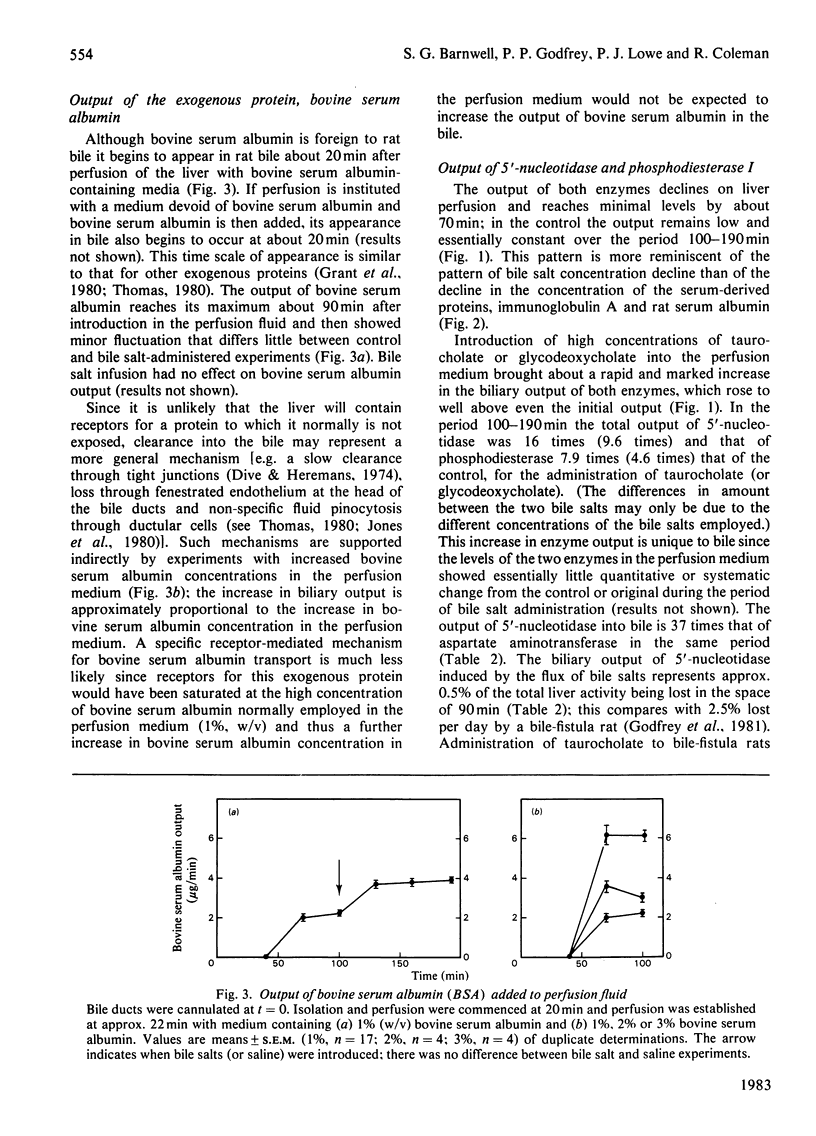
The output of proteins into bile was studied by using isolated perfused rat livers. Replacement of rat blood with defined perfusion media deprived the liver of rat serum proteins (albumin, immunoglobulin A) and resulted in a rapid decline in the amounts of these proteins in bile. When bovine serum albumin was incorporated into the perfusion medium it appeared in bile within 20 min and the amount in the bile was determined by the concentration of the protein in the perfusion medium. The use of a defined perfusion medium also deprived the livers of bile salts and the amounts of these, and of plasma-membrane enzymes [5'-nucleotidase (EC 3.1.3.5) and phosphodiesterase I], in bile declined rapidly. Introduction of micelle-forming bile salts (taurocholate or glycodeoxycholate) to the perfusion medium 80 min after liver isolation markedly increased the output of plasma-membrane enzymes but had no effect on the other proteins. The magnitude of this response was dependent on the bile salt used and its concentration in bile; there was little effect on plasma-membrane enzyme output until the critical micellar concentration of the bile salt had been exceeded in the bile. A bile salt analogue, taurodehydrocholate, which does not form micelles, did not produce the enhanced output of plasma-membrane enzymes. This work supports the view that the output of plasma-membrane enzymes in bile is a consequence of bile salt output and also provides evidence for mechanisms by which serum proteins enter the bile.
Full text
PDF

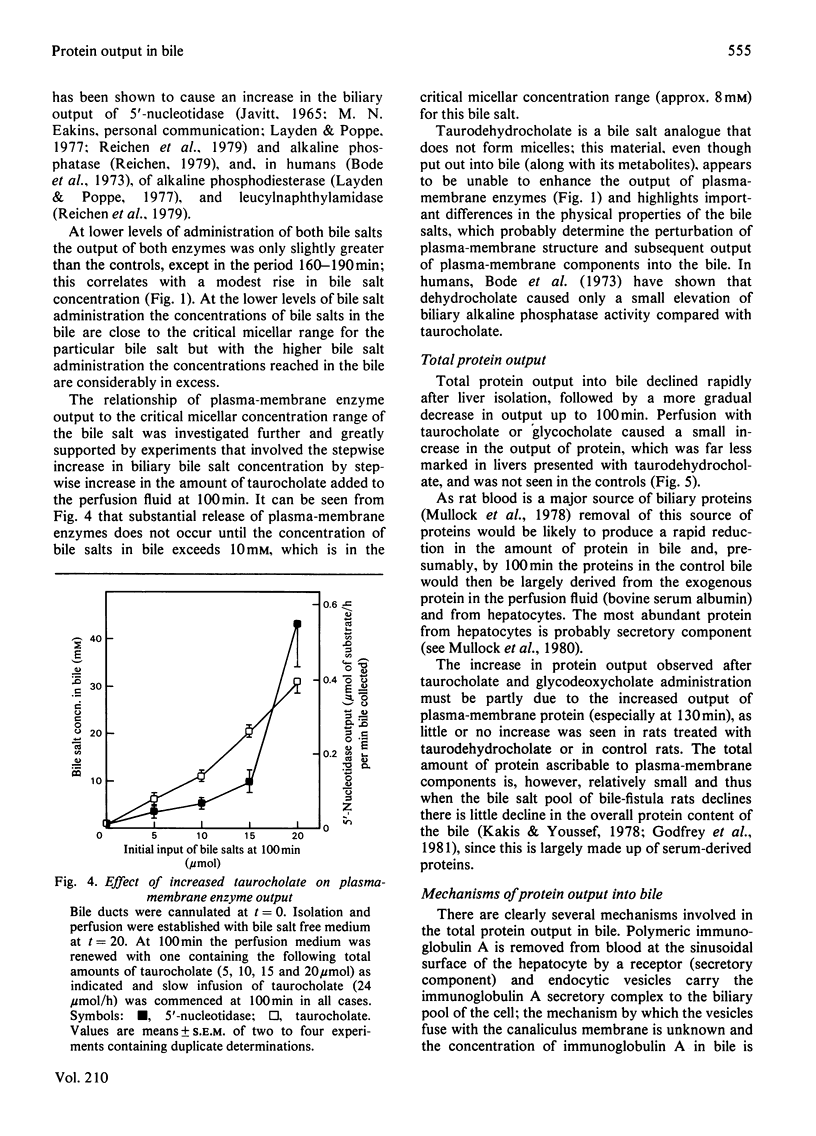
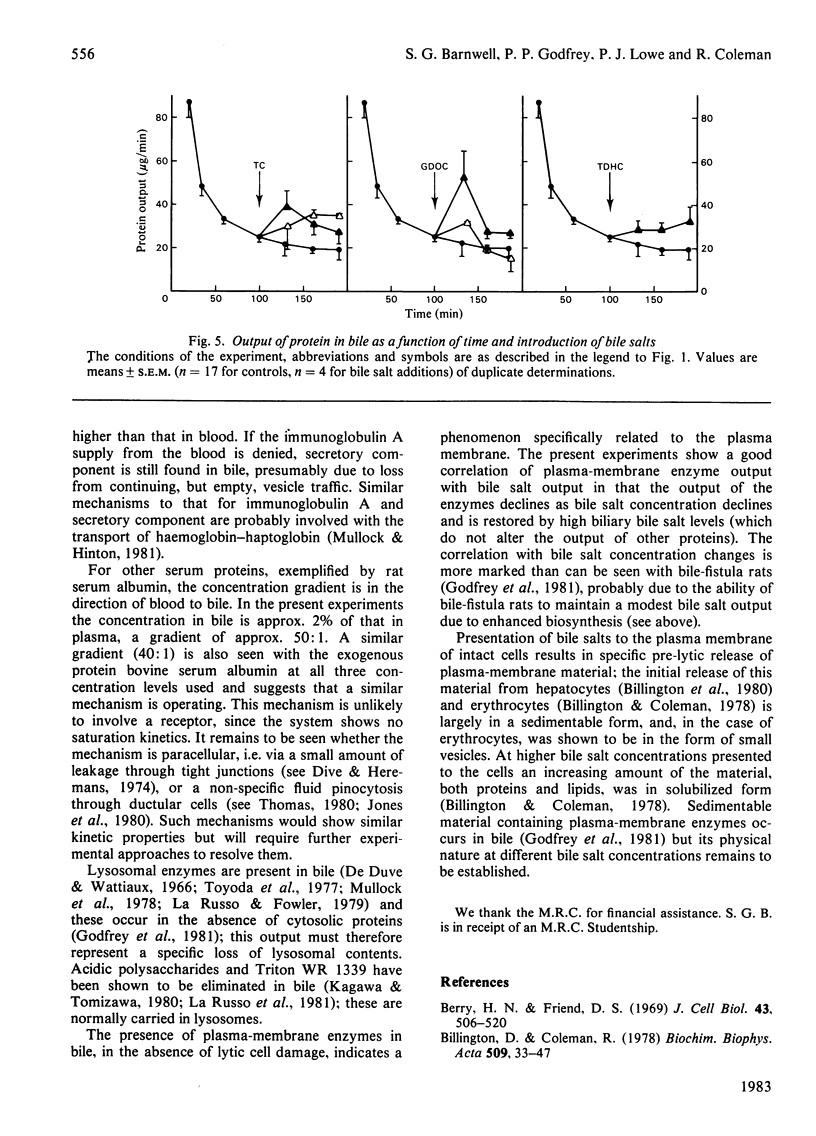

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAUER R. W., LEONG G. F., HOLLOWAY R. J. Mechanics of bile secretion; effect of perfusion pressure and temperature on bile flow and bile secretion pressure. Am J Physiol. 1954 Apr;177(1):103–112. doi: 10.1152/ajplegacy.1954.177.1.103. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billington D., Coleman R. Effects of bile salts of human erythrocytes. Plasma membrane vesiculation, phospholipid solubilization and their possible relationships to bile secretion. Biochim Biophys Acta. 1978 May 4;509(1):33–47. doi: 10.1016/0005-2736(78)90005-6. [DOI] [PubMed] [Google Scholar]
- Billington D., Evans C. E., Godfrey P. P., Coleman R. Effects of bile salts on the plasma membranes of isolated rat hepatocytes. Biochem J. 1980 May 15;188(2):321–327. doi: 10.1042/bj1880321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode J. C., Zelder O., Neuberger H. O. Effect of taurocholate, dehydrocholate and secretin on biliary output of alkaline phosphatase and GOT. Helv Med Acta. 1973 Sep;37(2):143–151. [PubMed] [Google Scholar]
- Coleman R., Iqbal S., Godfrey P. P., Billington D. Membranes and bile formation. Composition of several mammalian biles and their membrane-damaging properties. Biochem J. 1979 Jan 15;178(1):201–208. doi: 10.1042/bj1780201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. G., Skibba J. L. Improved in situ rat liver perfusion technique. J Surg Res. 1980 Jan;28(1):65–70. doi: 10.1016/0022-4804(80)90084-0. [DOI] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- Dive C., Heremans J. F. Nature and origin of the proteins of bile. I. A comparative analysis of serum and bile proteins in man. Eur J Clin Invest. 1974 Aug;4(4):235–239. doi: 10.1111/j.1365-2362.1974.tb00398.x. [DOI] [PubMed] [Google Scholar]
- Dive C., Nadalini R. A., Vaerman J. P., Heremans J. F. Origin and nature of the proteins of bile. II. A comparative analysis of serum, hepatic lymph and bile proteins in the dog. Eur J Clin Invest. 1974 Aug;4(4):241–246. doi: 10.1111/j.1365-2362.1974.tb00399.x. [DOI] [PubMed] [Google Scholar]
- Godfrey P. P., Lembra L., Coleman R. Effects of colchicine and vinblastine on output of proteins into bile. Biochem J. 1982 Oct 15;208(1):153–157. doi: 10.1042/bj2080153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey P. P., Warner M. J., Coleman R. Enzymes and proteins in bile. Variations in output in rat cannula bile during and after depletion of the bile-salt pool. Biochem J. 1981 Apr 15;196(1):11–16. doi: 10.1042/bj1960011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant D. A., Jones P. A., Magee A. I., Hermon-Taylor J. The biliary excretion of intravenously administered enterokinase [proceedings]. Biochem Soc Trans. 1980 Feb;8(1):55–55. doi: 10.1042/bst0080055. [DOI] [PubMed] [Google Scholar]
- Hinton R. H., Dobrota M., Mullock B. M. Haptoglobin-mediated transfer of haemoglobin from serum into bile. FEBS Lett. 1980 Apr 7;112(2):247–250. doi: 10.1016/0014-5793(80)80190-6. [DOI] [PubMed] [Google Scholar]
- Holdsworth G., Coleman R. Enzyme profiles of mammalian bile. Biochim Biophys Acta. 1975 Apr 21;389(1):47–50. doi: 10.1016/0005-2736(75)90384-3. [DOI] [PubMed] [Google Scholar]
- Jones A. L., Schmucker D. L., Renston R. H., Murakami T. The architecture of bile secretion. A morphological perspective of physiology. Dig Dis Sci. 1980 Aug;25(8):609–629. doi: 10.1007/BF01318875. [DOI] [PubMed] [Google Scholar]
- Kagawa K., Tomizawa S. Exocytotic excretion of dextran sulfates from liver to bile. Jpn J Pharmacol. 1980 Feb;30(1):101–108. doi: 10.1254/jjp.30.101. [DOI] [PubMed] [Google Scholar]
- Kakis G., Yousef I. M. Protein composition of rat bile. Can J Biochem. 1978 May;56(5):287–290. doi: 10.1139/o78-044. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaRusso N. F., Fowler S. Coordinate secretion of acid hydrolases in rat bile. J Clin Invest. 1979 Oct;64(4):948–954. doi: 10.1172/JCI109561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Mullock B. M., Dobrota M., Hinton R. H. Sources of the proteins of rat bile. Biochim Biophys Acta. 1978 Nov 1;543(4):497–507. doi: 10.1016/0304-4165(78)90304-5. [DOI] [PubMed] [Google Scholar]
- Mullock B. M., Jones R. S., Hinton R. H. Movement of endocytic shuttle vesicles from the sinusoidal to the bile canalicular face of hepatocytes does not depend on occupation of receptor sites. FEBS Lett. 1980 May 5;113(2):201–205. doi: 10.1016/0014-5793(80)80591-6. [DOI] [PubMed] [Google Scholar]
- Russo N., Cipolletta L., Diadema M. R., Parente A., Lonardo L. Validità diagnostica del Cu e del Mg nelle emolinfopatie e nei tumori solidi. Minerva Med. 1981 May 26;72(21):1331–1338. [PubMed] [Google Scholar]
- Thomas P. Studies on the mechanisms of biliary excretion of circulating glycoproteins. The carcinoembryonic antigen. Biochem J. 1980 Dec 15;192(3):837–843. doi: 10.1042/bj1920837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda S., Eto Y., Aoki K. Bile lysosomal enzymes: characteristics and pathological significance for various hepatobiliary disorders. Clin Chim Acta. 1977 Sep 1;79(2):291–298. doi: 10.1016/0009-8981(77)90421-1. [DOI] [PubMed] [Google Scholar]


