Abstract
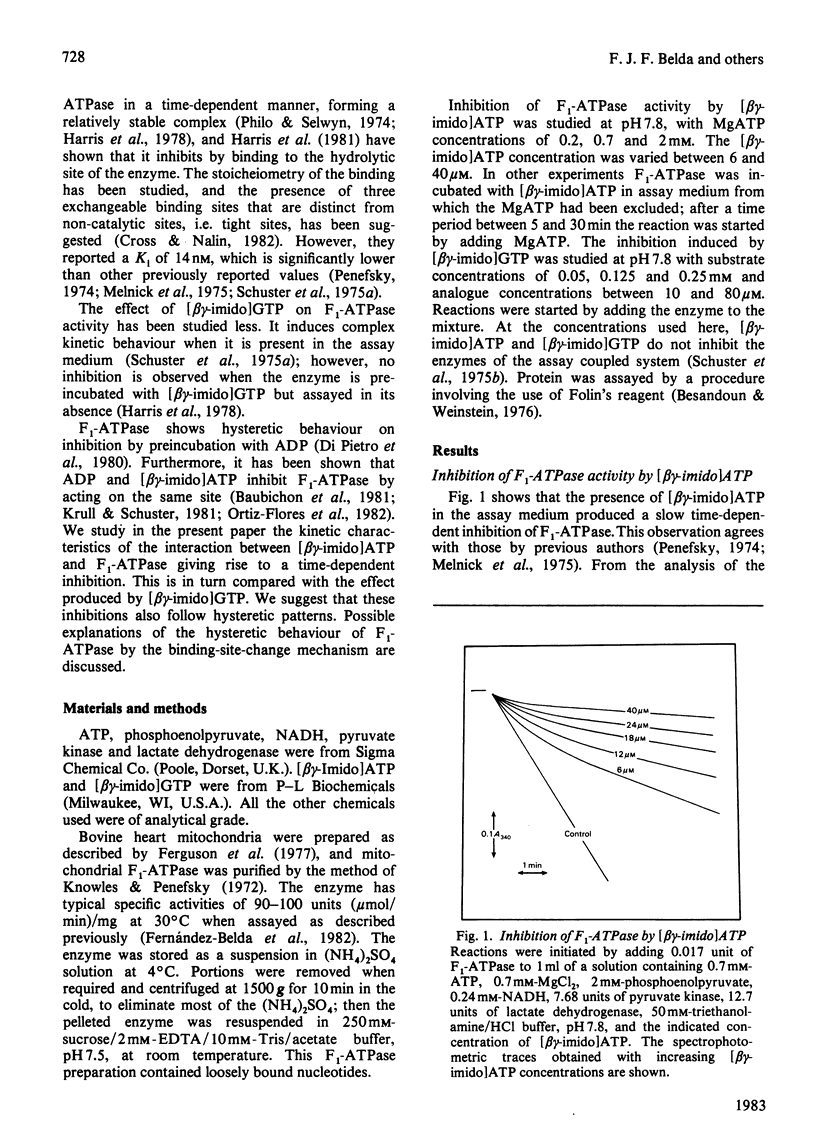
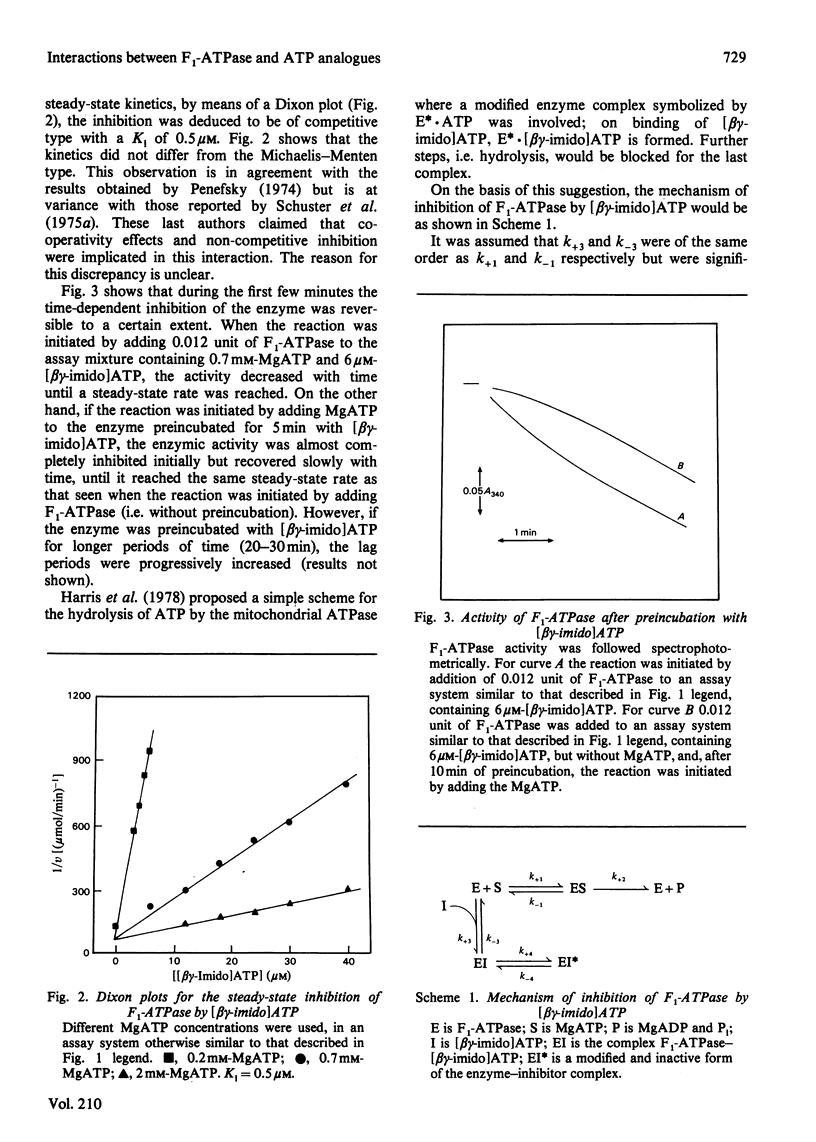
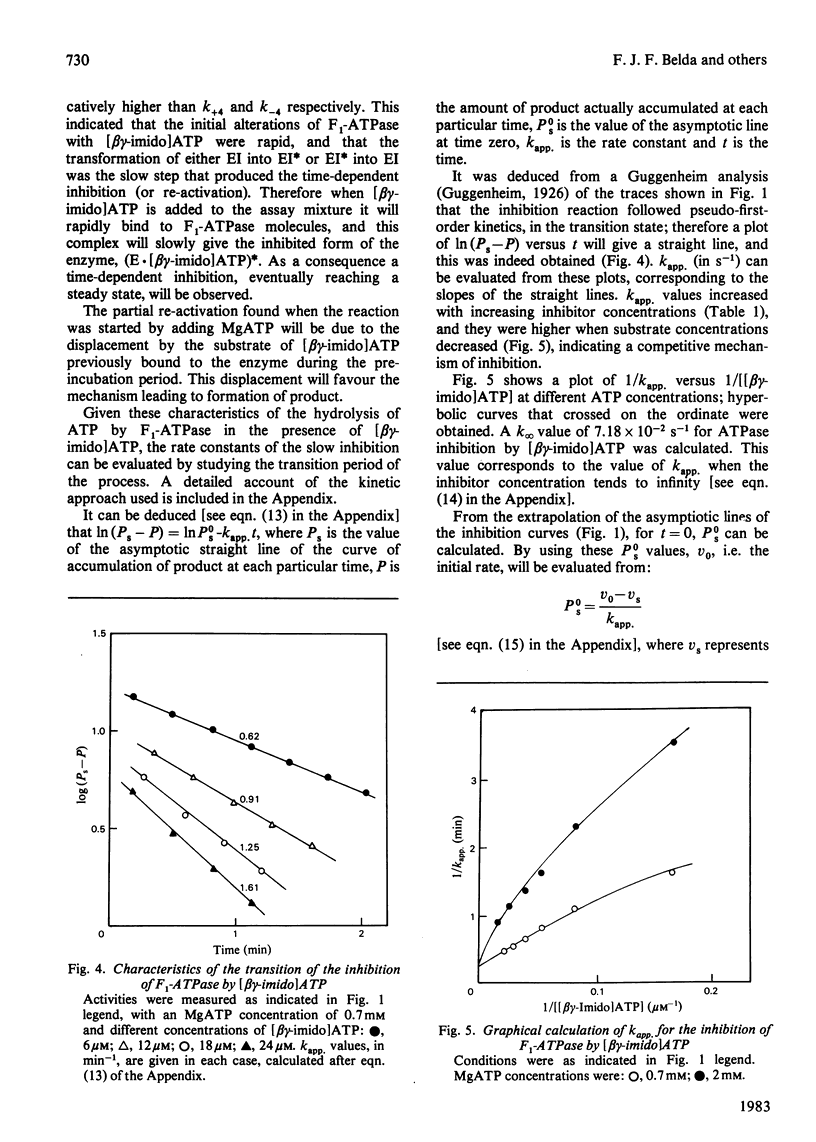
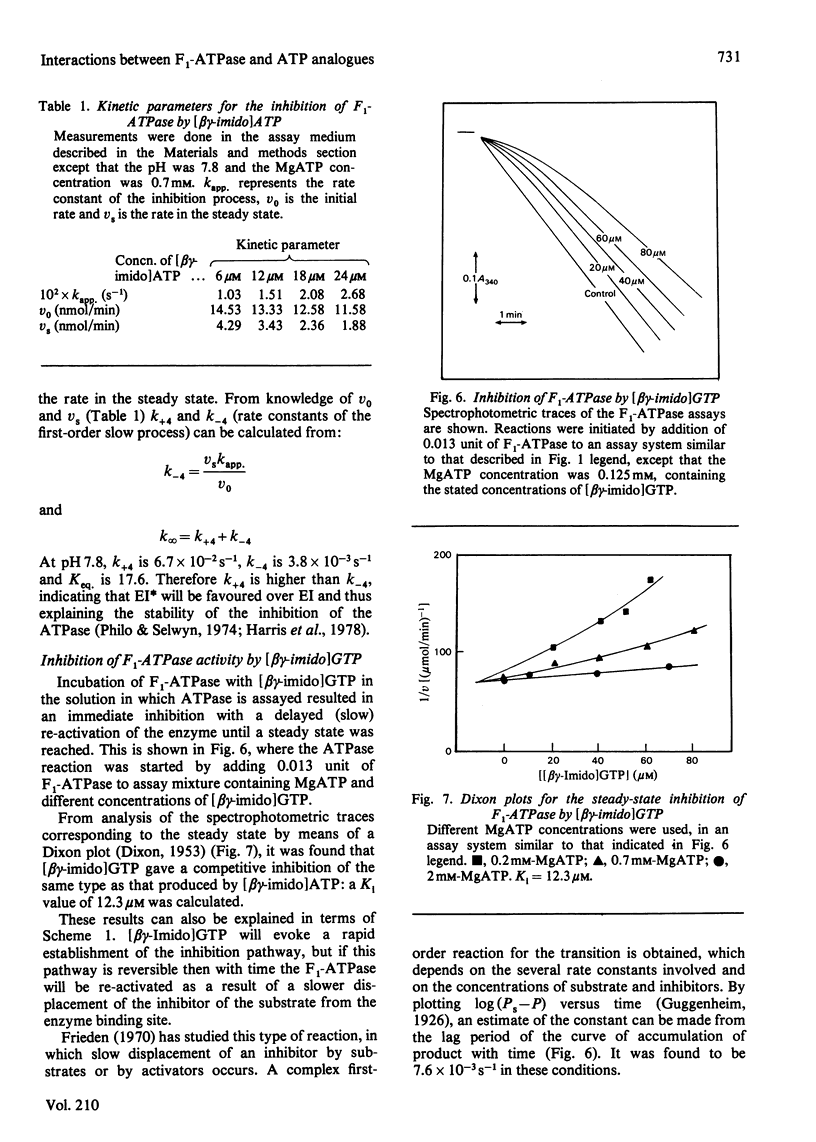
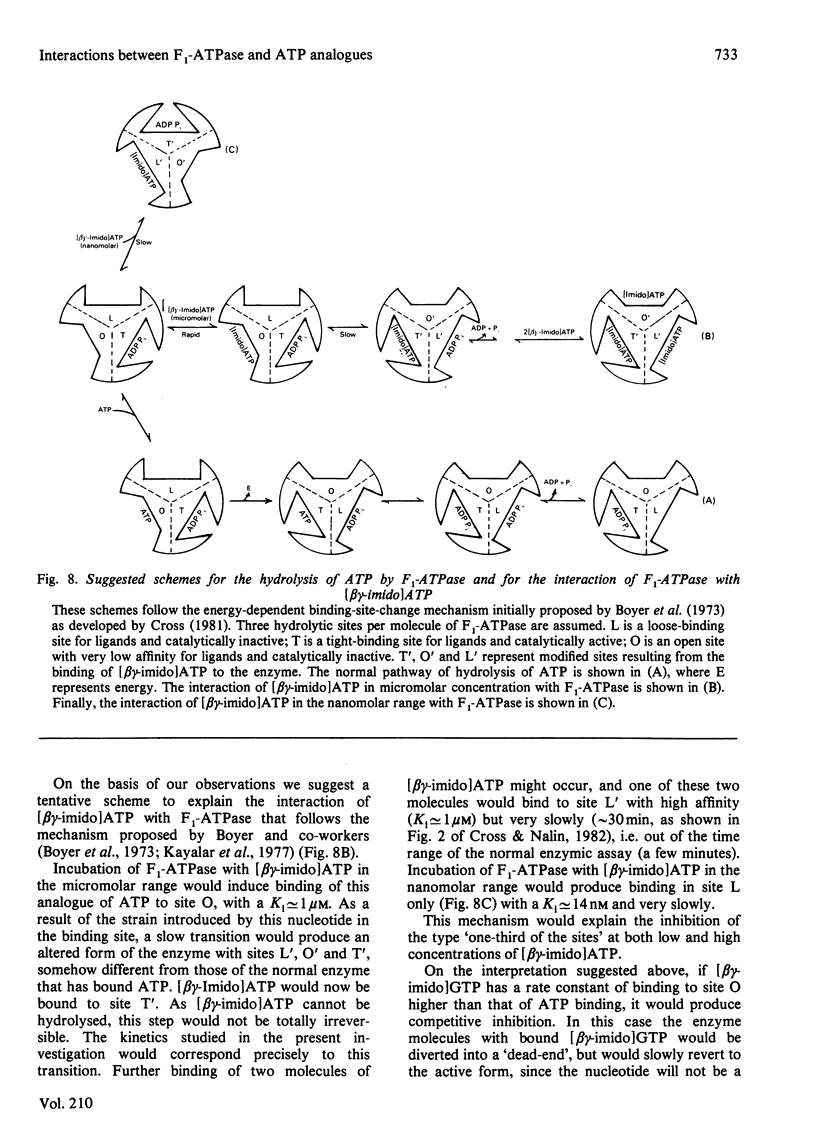
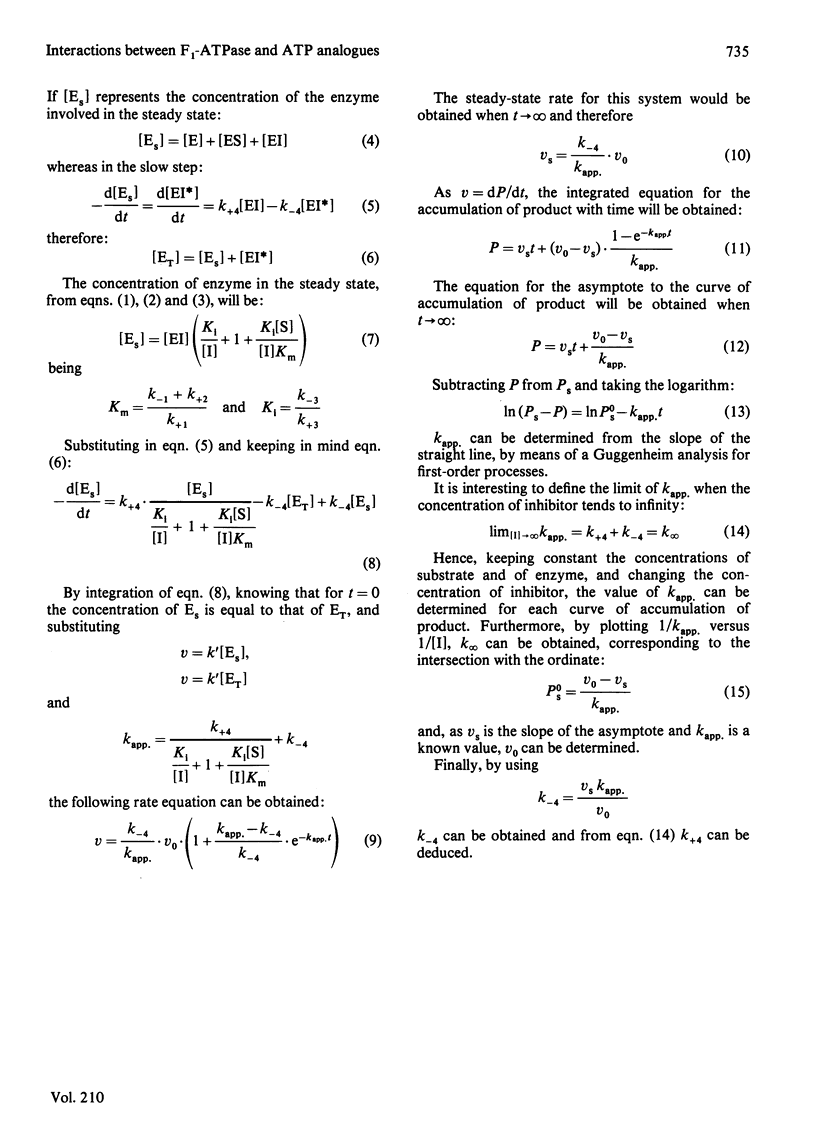
1. The presence of 5'-adenylyl imidodiphosphate, a non-hydrolysable analogue of ATP, in the solution used to assay the soluble bovine heart mitochondrial F1-ATPase produced slow competitive inhibition. If the enzyme was preincubated with the inhibitor before the substrate, MgATP, was added, a partial re-activation was obtained. 2. The slow inhibitory process showed first-order rate kinetics, and therefore it seems likely that a conformational change of the enzyme occurs following a faster binding process. A reaction scheme is suggested. At pH 7.8 the rate constant for the inhibition reaction was calculated to be 6.7 X 10(-2)s-1 and that for the re-activation 3.8 X 10(-3)s-1, with Keq. 17.6, indicating that the inhibited enzyme-inhibitor complex will be favoured over the non-inhibited enzyme-inhibitor complex. 3. The presence of 5'-guanylyl imidodiphosphate in the solution used to assay F1-ATPase produced rapid competitive inhibition, which was then slowly reversed until a steady state was reached. This might be explained by a rapid but reversible shift of the inhibition pathway induced by this non-hydrolysable analogue of ATP. A complex rate constant for the displacement of the inhibitor by the substrate of 7.6 X 10(-3)s-1 was calculated. 4. The results are discussed in the light of other recent observations about binding of 5'-adenylyl imidodiphosphate to F1-ATPase and with reference to the binding-site-change mechanism of hydrolysis of ATP by F1-ATPase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baubichon H., Godinot C., Di Pietro A., Gautheron D. C. Competition between ADP and nucleotide analogues to occupy regulatory sites(s) related to hysteretic inhibition of mitochondrial F1-ATPase. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1032–1038. doi: 10.1016/0006-291x(81)91927-6. [DOI] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Boyer P. D., Cross R. L., Momsen W. A new concept for energy coupling in oxidative phosphorylation based on a molecular explanation of the oxygen exchange reactions. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2837–2839. doi: 10.1073/pnas.70.10.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyak B. C., Kozlov I. A. Adenylylimidodiphosphate release from the active site of submitochondrial particles ATPase. FEBS Lett. 1979 Aug 15;104(2):215–219. doi: 10.1016/0014-5793(79)80817-0. [DOI] [PubMed] [Google Scholar]
- Cross R. L., Nalin C. M. Adenine nucleotide binding sites on beef heart F1-ATPase. Evidence for three exchangeable sites that are distinct from three noncatalytic sites. J Biol Chem. 1982 Mar 25;257(6):2874–2881. [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pietro A., Penin F., Godinot C., Gautheron D. C. "Hysteric" behavior and nucleotide binding sites of pig heart mitochondrial F1 adenosine 5'-triphosphatase. Biochemistry. 1980 Dec 9;19(25):5671–5678. doi: 10.1021/bi00566a002. [DOI] [PubMed] [Google Scholar]
- Ferguson S. J., Harris D. A., Radda G. K. The adenosine triphosphatase-inhibitor content of bovine heart submitochondrial particles. Influence of the inhibitor on adenosine triphosphate-dependent reactions. Biochem J. 1977 Feb 15;162(2):351–357. doi: 10.1042/bj1620351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Belda F. J., García-Carmona F., García-Cánovas F., Lozano J. A., Gómez-Fernández J. C. Mitochondrial ATPase inactivation by interaction with its substrate. Arch Biochem Biophys. 1982 Apr 15;215(1):40–46. doi: 10.1016/0003-9861(82)90276-4. [DOI] [PubMed] [Google Scholar]
- Flores G. O., Acosta A., Puyou A. G. Characteristics of adenylyl imidodiphosphate- and ADP-binding sites insoluble and particulate mitochondrial ATPase. Studies with methanol. Biochim Biophys Acta. 1982 Mar 16;679(3):466–473. doi: 10.1016/0005-2728(82)90168-2. [DOI] [PubMed] [Google Scholar]
- Frieden C. Kinetic aspects of regulation of metabolic processes. The hysteretic enzyme concept. J Biol Chem. 1970 Nov 10;245(21):5788–5799. [PubMed] [Google Scholar]
- Harris D. A., Baltscheffsky M. Bound nucleotides and phosphorylation in Rhodospirillum rubrum. Biochem Biophys Res Commun. 1979 Feb 28;86(4):1248–1255. doi: 10.1016/0006-291x(79)90251-1. [DOI] [PubMed] [Google Scholar]
- Harris D. A., Dall-Larsen T., Klungsøyr L. Studies of the kinetics of the isolated mitochondrial ATPase using dinitrophenol as a probe. Biochim Biophys Acta. 1981 Apr 13;635(2):412–418. doi: 10.1016/0005-2728(81)90039-6. [DOI] [PubMed] [Google Scholar]
- Harris D. A., Gomez-Fernandez J. C., Klungsøyr L., Radda G. K. Specificity of nucleotide binding and coupled reactions utilising the mitochondrial ATPase. Biochim Biophys Acta. 1978 Dec 7;504(3):364–383. doi: 10.1016/0005-2728(78)90060-9. [DOI] [PubMed] [Google Scholar]
- Harris D. A. The interactions of coupling ATPases with nucleotides. Biochim Biophys Acta. 1978 Mar 10;463(3-4):245–273. doi: 10.1016/0304-4173(78)90002-2. [DOI] [PubMed] [Google Scholar]
- Kayalar C., Rosing J., Boyer P. D. An alternating site sequence for oxidative phosphorylation suggested by measurement of substrate binding patterns and exchange reaction inhibitions. J Biol Chem. 1977 Apr 25;252(8):2486–2491. [PubMed] [Google Scholar]
- Knowles A. F., Penefsky H. S. The subunit structure of beef heart mitochondrial adenosine triphosphatase. Isolation procedures. J Biol Chem. 1972 Oct 25;247(20):6617–6623. [PubMed] [Google Scholar]
- Krull K. W., Schuster S. M. Kinetic studies of beef heart mitochondrial adenosine triphosphatase: interaction of the inhibitor protein and adenosine triphosphate analogues. Biochemistry. 1981 Mar 17;20(6):1592–1598. doi: 10.1021/bi00509a028. [DOI] [PubMed] [Google Scholar]
- Melnick R. L., De Sousa J. T., Magiure J., Packer L. Action of the adenosine triphosphate analog, adenylyl imidodiphosphate in mitochondria. Arch Biochem Biophys. 1975 Jan;166(1):139–144. doi: 10.1016/0003-9861(75)90373-2. [DOI] [PubMed] [Google Scholar]
- Penefsky H. S. Differential effects of adenylyl imidodiphosphate on adenosine triphosphate synthesis and the partial reactions of oxidative phosphorylation. J Biol Chem. 1974 Jun 10;249(11):3579–3585. [PubMed] [Google Scholar]
- Penefsky H. S. Mitochondrial ATPase. Adv Enzymol Relat Areas Mol Biol. 1979;49:223–280. doi: 10.1002/9780470122945.ch6. [DOI] [PubMed] [Google Scholar]
- Philo R. D., Selwyn M. J. Inhibition of the soluble adenosine triphosphatase from mitochondria by adenylyl imidodiphosphate. Biochem J. 1974 Dec;143(3):745–749. doi: 10.1042/bj1430745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster S. M., Ebel R. E., Lardy H. A. Kinetic studies on rat liver and beef heart mitochondrial ATPase. Evidence for nucleotide binding at separate regulatory and catalytic sites. J Biol Chem. 1975 Oct 10;250(19):7848–7853. [PubMed] [Google Scholar]
- Schuster S. M., Ebel R. E., Lardy H. A. Kinetic studies on rat liver and beef heart mitochondrial adenosine triphosphatase: the effects of the chromium complexes of adenosine triposphate and adenosine diphosphate on the kinetic properties. Arch Biochem Biophys. 1975 Dec;171(2):656–661. doi: 10.1016/0003-9861(75)90077-6. [DOI] [PubMed] [Google Scholar]
- Slater E. C., Kemp A., van der Kraan I., Muller J. L., Roveri O. A., Verschoor G. J., Wagenvoord R. J., Wielders J. P. The ATP-and ADP-binding sites in mitochondrial coupling factor F1 and their possible role in oxidative phosphorylation. FEBS Lett. 1979 Jul 1;103(1):7–11. doi: 10.1016/0014-5793(79)81239-9. [DOI] [PubMed] [Google Scholar]
- Tondre C., Hammes G. G. A kinetic study of the binding of an ADP fluorescent analog to mitochondrial ATPase. Biochim Biophys Acta. 1973 Aug 31;314(2):245–249. doi: 10.1016/0005-2728(73)90139-4. [DOI] [PubMed] [Google Scholar]
- Yount R. G., Babcock D., Ballantyne W., Ojala D. Adenylyl imidodiphosphate, an adenosine triphosphate analog containing a P--N--P linkage. Biochemistry. 1971 Jun 22;10(13):2484–2489. doi: 10.1021/bi00789a009. [DOI] [PubMed] [Google Scholar]


