Abstract
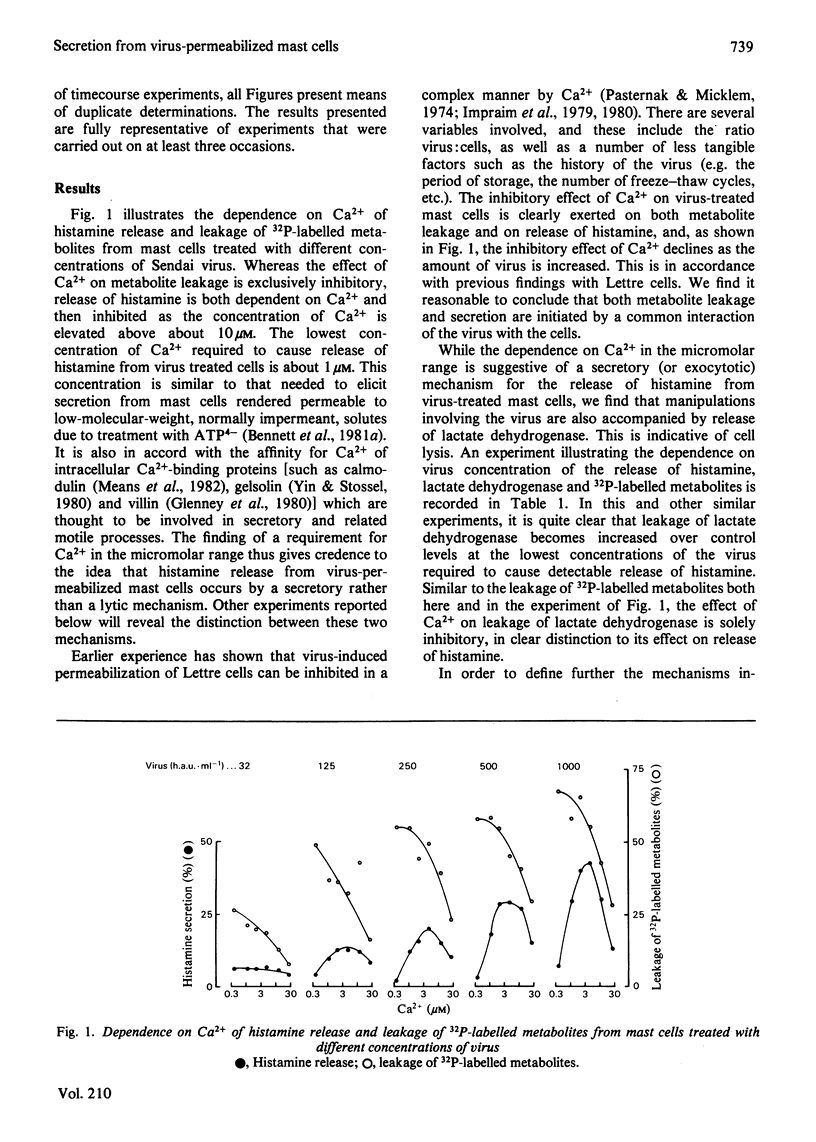
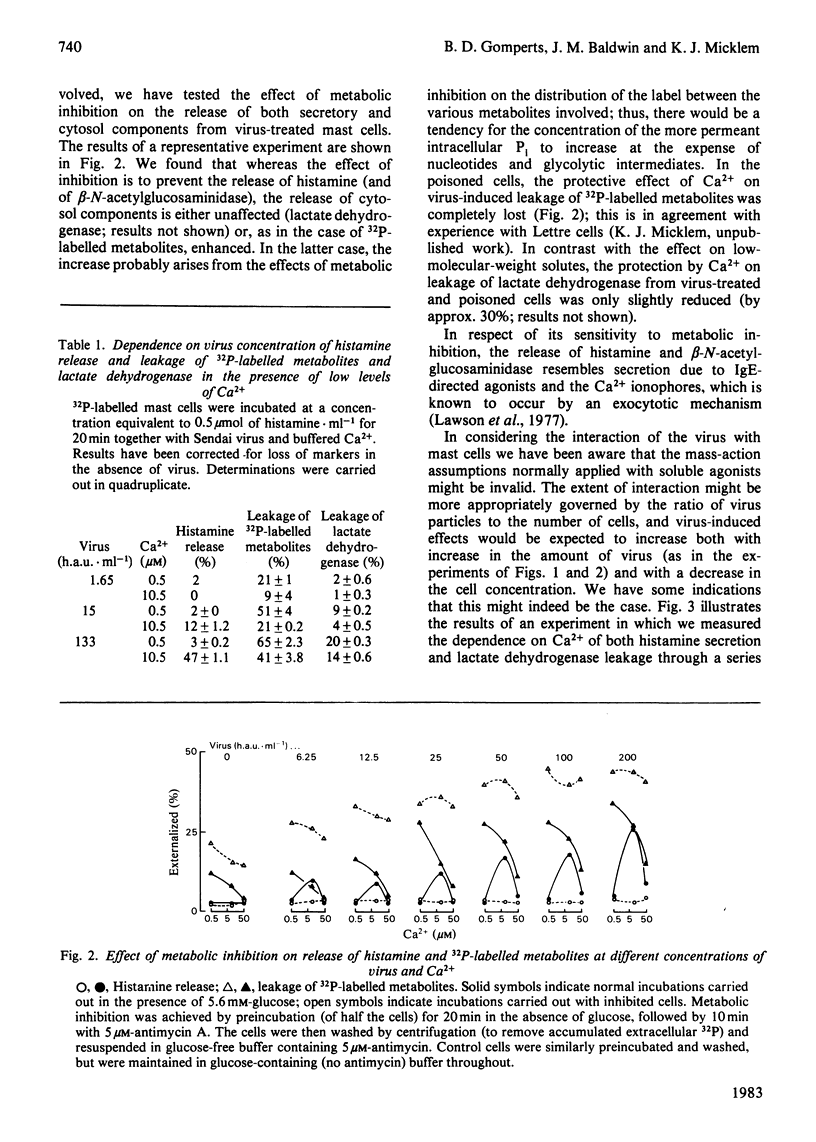
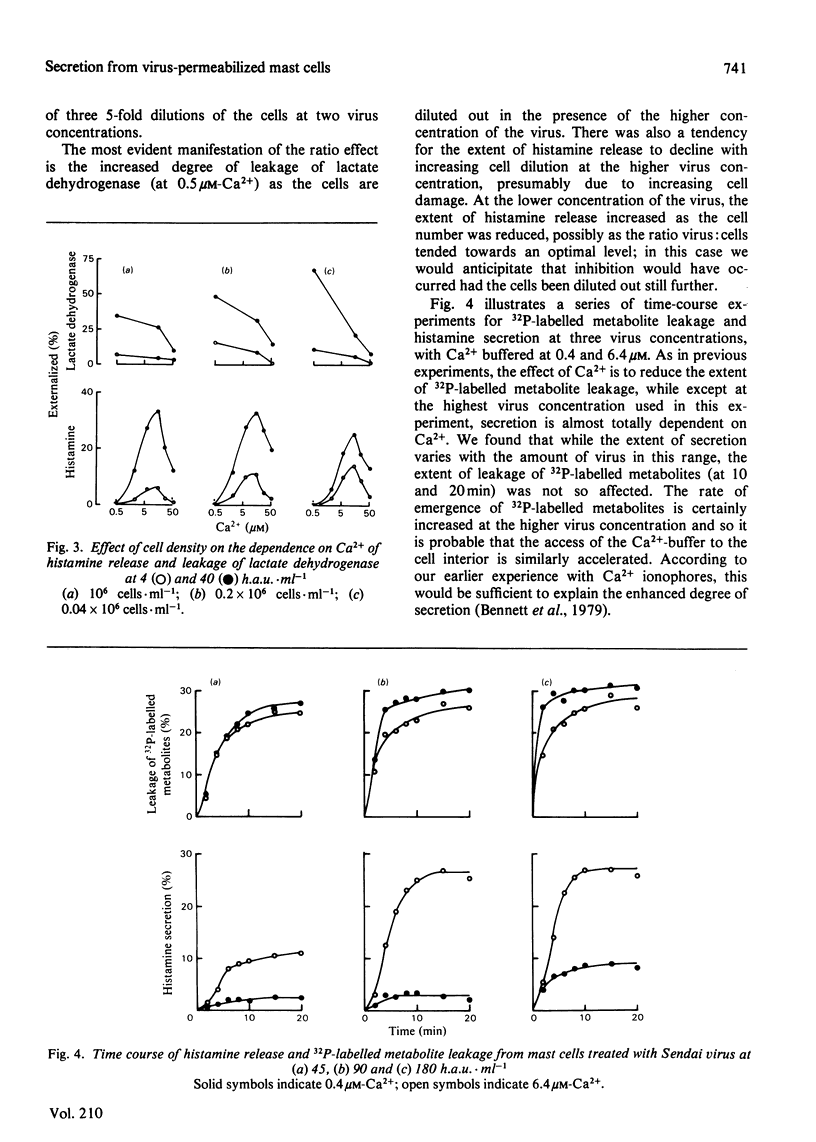
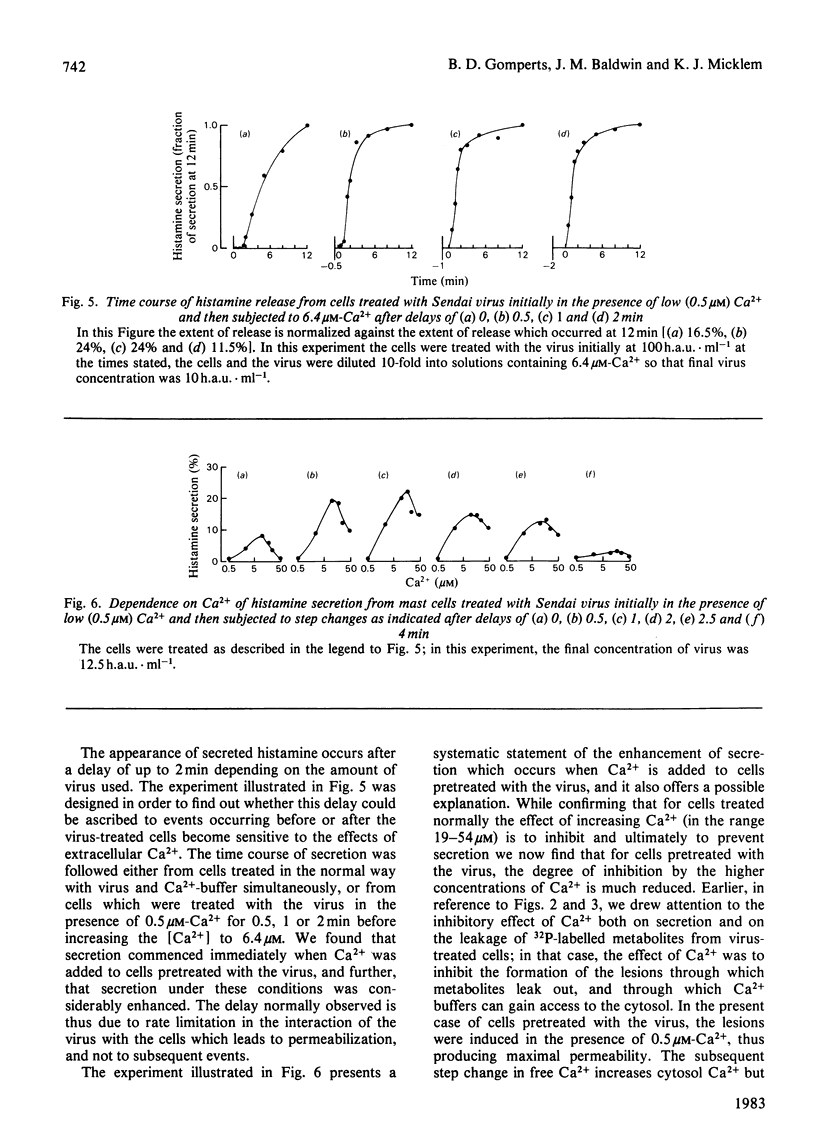
In the presence of low extracellular Ca2+, Sendai virus generates permeability lesions in the membrane of rat mast cells. This causes leakage of intracellular phosphorylated metabolites and lactate dehydrogenase; it also permits uptake of normally impermeant aqueous solutes such as the complex of Ca2+ with N-hydroxyethylethylenediaminetriacetic acid. We have used this system to buffer the concentration of cytosol Ca2+ in the micromolar range and thus to cause release of the contents of the secretory granules such as histamine and beta-N-acetylglucosaminidase. We argue that such release occurs by a normal exocytotic secretory mechanism for the following reasons. (1) While leakage of cytosol components is progressively inhibited by Ca2+ in the range 1-10 microM, release of histamine is dependent on Ca2+ in this range. Higher concentrations of Ca2+ are inhibitory to both permeabilization and histamine release. (2) While leakage of phosphorylated metabolites is enhanced in metabolically inhibited cells and the leakage of lactate dehydrogenase is insensitive to metabolic inhibition, the release of histamine and beta-N-acetylglucosaminidase is strictly dependent on an intact metabolic process.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Knight D. E. Calcium control of exocytosis and endocytosis in bovine adrenal medullary cells. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):83–103. doi: 10.1098/rstb.1981.0174. [DOI] [PubMed] [Google Scholar]
- Bennett J. P., Cockcroft S., Gomperts B. D. Ionomycin stimulates mast cell histamine secretion by forming a lipid-soluble calcium complex. Nature. 1979 Dec 20;282(5741):851–853. doi: 10.1038/282851a0. [DOI] [PubMed] [Google Scholar]
- Bennett J. P., Cockcroft S., Gomperts B. D. Rat mast cells permeabilized with ATP secrete histamine in response to calcium ions buffered in the micromolar range. J Physiol. 1981 Aug;317:335–345. doi: 10.1113/jphysiol.1981.sp013828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom G. D., Haegermark O. Studies on morphological changes and histamine release induced by bee venom, n-decylamine and hypotonic solutions in rat peritoneal mast cells. Acta Physiol Scand. 1967 Dec;71(4):257–269. doi: 10.1111/j.1748-1716.1967.tb03733.x. [DOI] [PubMed] [Google Scholar]
- Bächi T., Aguet M., Howe C. Fusion of erythrocytes by Sendai virus studied by immuno-freeze-etching. J Virol. 1973 Jun;11(6):1004–1012. doi: 10.1128/jvi.11.6.1004-1012.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. ATP induces nucleotide permeability in rat mast cells. Nature. 1979 Jun 7;279(5713):541–542. doi: 10.1038/279541a0. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Activation and inhibition of calcium-dependent histamine secretion by ATP ions applied to rat mast cells. J Physiol. 1979 Nov;296:229–243. doi: 10.1113/jphysiol.1979.sp013002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. The ATP4- receptor of rat mast cells. Biochem J. 1980 Jun 15;188(3):789–798. doi: 10.1042/bj1880789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVENPORT F. M., ROTT R., SCHAEFER W. Physical and biological properties of influenza virus components obtained after ether treatment. J Exp Med. 1960 Nov 1;112:765–782. doi: 10.1084/jem.112.5.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewtrell C. M., Gomperts B. D. Effect of flavone inhibitors of transport ATPases on histamine secretion from rat mast cells. Nature. 1977 Feb 17;265(5595):635–636. doi: 10.1038/265635a0. [DOI] [PubMed] [Google Scholar]
- Flowers A. L., Hessinger D. A. Mast cell histamine release induced by Portuguese man-of-war (Physalia) venom. Biochem Biophys Res Commun. 1981 Dec 15;103(3):1083–1091. doi: 10.1016/0006-291x(81)90919-0. [DOI] [PubMed] [Google Scholar]
- Forda S. R., Gillies G., Kelly J. S., Micklem K. J., Pasternak C. A. Acute membrane responses to viral action. Neurosci Lett. 1982 Apr 26;29(3):237–242. doi: 10.1016/0304-3940(82)90323-8. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L., Gomperts B. D. Calcium ionophores and movement of calcium ions following the physiological stimulus to a secretory process. Nature. 1973 Oct 5;245(5423):249–251. doi: 10.1038/245249a0. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L. The role of the alkaline earth ions in anaphylactic histamine secretion. J Physiol. 1972 Aug;224(3):753–769. doi: 10.1113/jphysiol.1972.sp009921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Bretscher A., Weber K. Calcium control of the intestinal microvillus cytoskeleton: its implications for the regulation of microfilament organizations. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6458–6462. doi: 10.1073/pnas.77.11.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomperts B. D., Bennett J. P., Allan D. A synthetic ionophore for Ca2+: studies with model and biological systems. Eur J Biochem. 1981 Jul;117(3):559–562. doi: 10.1111/j.1432-1033.1981.tb06373.x. [DOI] [PubMed] [Google Scholar]
- Goth A., Adams H. R., Knoohuizen M. Phosphatidylserine: selective enhancer of histamine release. Science. 1971 Sep 10;173(4001):1034–1035. doi: 10.1126/science.173.4001.1034. [DOI] [PubMed] [Google Scholar]
- Impraim C. C., Foster K. A., Micklem K. J., Pasternak C. A. Nature of virally mediated changes in membrane permeability to small molecules. Biochem J. 1980 Mar 15;186(3):847–860. doi: 10.1042/bj1860847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impraim C. C., Micklem K. J., Pasternak C. A. Calcium, cells and virus--alterations caused by paramyxoviruses. Biochem Pharmacol. 1979 Jun 15;28(12):1963–1969. doi: 10.1016/0006-2952(79)90652-x. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Hallam T. J., Scrutton M. C. Agonist selectivity and second messenger concentration in Ca2+-mediated secretion. Nature. 1982 Mar 18;296(5854):256–257. doi: 10.1038/296256a0. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Scrutton M. C. Direct evidence for a role for Ca2+ in amine storage granule secretion by human platelets. Thromb Res. 1980 Nov 15;20(4):437–446. doi: 10.1016/0049-3848(80)90282-0. [DOI] [PubMed] [Google Scholar]
- Lawson D., Raff M. C., Gomperts B., Fewtrell C., Gilula N. B. Molecular events during membrane fusion. A study of exocytosis in rat peritoneal mast cells. J Cell Biol. 1977 Feb;72(2):242–259. doi: 10.1083/jcb.72.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONGAR J. L., SCHILD H. O. Effect of temperature on the anaphylactic reaction. J Physiol. 1957 Feb 15;135(2):320–338. doi: 10.1113/jphysiol.1957.sp005713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOTA I. Action of anaphylactic shock and anaphylatoxin on mast cells and histamine in rats. Br J Pharmacol Chemother. 1957 Dec;12(4):453–456. doi: 10.1111/j.1476-5381.1957.tb00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means A. R., Tash J. S., Chafouleas J. G. Physiological implications of the presence, distribution, and regulation of calmodulin in eukaryotic cells. Physiol Rev. 1982 Jan;62(1):1–39. doi: 10.1152/physrev.1982.62.1.1. [DOI] [PubMed] [Google Scholar]
- Pasternak C. A., Micklem K. J. Permeability changes during cell fusion. J Membr Biol. 1973;14(3):293–303. doi: 10.1007/BF01868082. [DOI] [PubMed] [Google Scholar]
- Pasternak C. A., Micklem K. J. The biochemistry of virus-induced cell fusion. Changes in membrane integrity. Biochem J. 1974 Jun;140(3):405–411. doi: 10.1042/bj1400405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak C. A., Micklem K. J. Virally induced alterations in cellular permeability: a basis of cellular and physiological damage? Biosci Rep. 1981 Jun;1(6):431–448. doi: 10.1007/BF01121577. [DOI] [PubMed] [Google Scholar]
- Sugiyama K. Histamine release from rat mast cells induced by Sendai virus. Nature. 1977 Dec 15;270(5638):614–615. doi: 10.1038/270614a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature. 1981 Apr 9;290(5806):527–528. doi: 10.1038/290527a0. [DOI] [PubMed] [Google Scholar]
- Wyke A. M., Impraim C. C., Knutton S., Pasternak C. A. Components involved in virally mediated membrane fusion and permeability changes. Biochem J. 1980 Sep 15;190(3):625–638. doi: 10.1042/bj1900625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H. L., Stossel T. P. Purification and structural properties of gelsolin, a Ca2+-activated regulatory protein of macrophages. J Biol Chem. 1980 Oct 10;255(19):9490–9493. [PubMed] [Google Scholar]


