Abstract
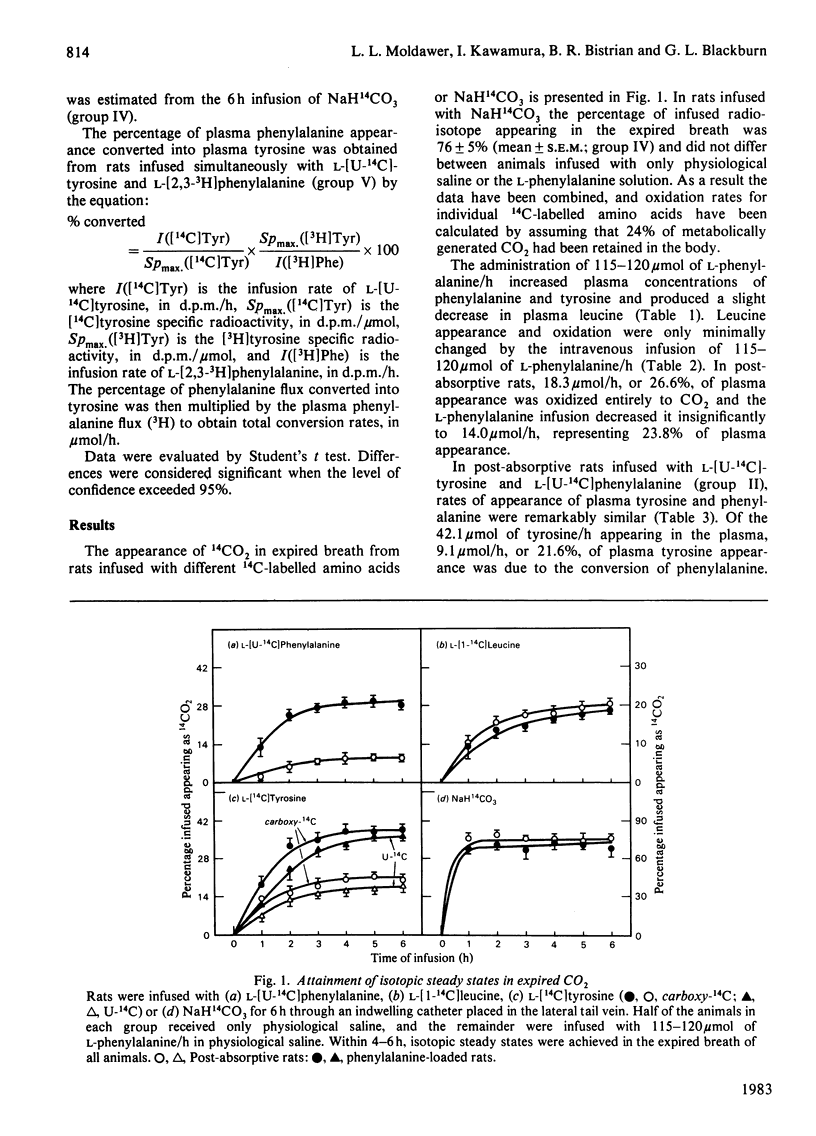
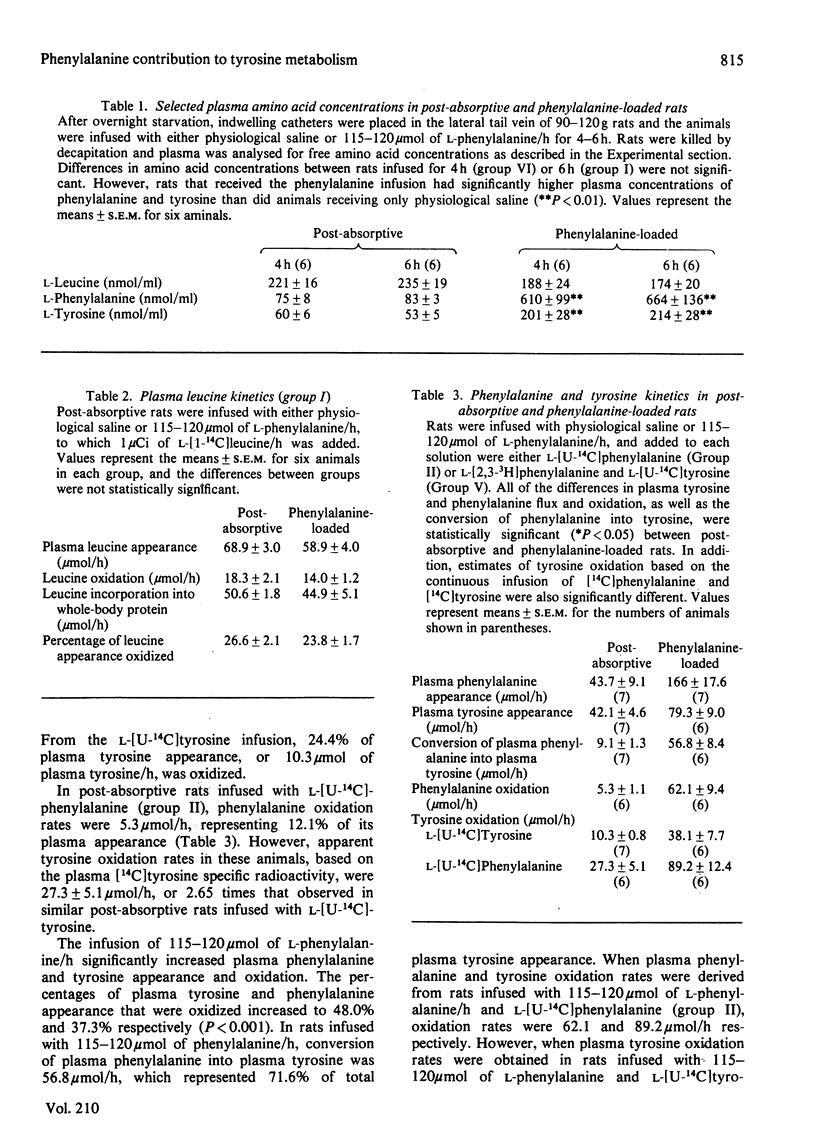
1. Rates of appearance and oxidation of plasma L-leucine, L-phenylalanine and L-tyrosine, as well as conversion of plasma phenylalanine into plasma tyrosine, were determined in 90-120 g rats after overnight starvation and while receiving 115-120 mumol of L-phenylalanine/h. 2. In the post-absorptive state, plasma tyrosine and phenylalanine appearances were similar, despite the fact that 22% of plasma tyrosine appearance could be attributed to the hydroxylation of phenylalanine. 3. A constant infusion of 115-120 mumol of L-phenylalanine/h did not significantly alter plasma leucine kinetics, but increased appearance of plasma phenylalanine and tyrosine. The percentage of phenylalanine and tyrosine appearance that was oxidized increased from 12.1% and 24.4% to 37.3% and 48.0% respectively. In phenylalanine-loaded rats, 72% of plasma tyrosine appearance could be attributed to the conversion of phenylalanine. 4. Whole-body tyrosine oxidation measured from a continuous infusion of either L-[14C]tyrosine or L-[14C]phenylalanine differed by 165%. 5. It can be concluded that, in the post-absorptive state, phenylalanine hydroxylation makes a substantial contribution to the plasma appearance of tyrosine and is significantly increased when phenylalanine is administered. The disposal of excess infused phenylalanine is a result of a greater percentage of plasma phenylalanine being converted into tyrosine and a greater proportion of tyrosine being further oxidized. However, apparent tyrosine oxidation rates estimated from plasma tyrosine specific radioactivities and appearance of expired 14CO2 during administration of [14C]tyrosine are underestimates of true rates, in part because tyrosine generated from phenylalanine hydroxylation is catabolized without freely equilibrating with the plasma compartment.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURRILL L. M., SCHUCK C. PHENYLALANINE REQUIREMENTS WITH DIFFERENT LEVELS OF TYROSINE. J Nutr. 1964 Jul;83:202–208. doi: 10.1093/jn/83.3.202. [DOI] [PubMed] [Google Scholar]
- Brand L. M., Harper A. E. Effect of glucagon on phenylalanine metabolism and phenylalanine-degrading enzymes in the rat. Biochem J. 1974 Aug;142(2):231–245. doi: 10.1042/bj1420231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke J. T., Bier D. M. The conversion of phenylalanine to tyrosine in man. Direct measurement by continuous intravenous tracer infusions of L-[ring-2H5]phenylalanine and L-[1-13C] tyrosine in the postabsorptive state. Metabolism. 1982 Oct;31(10):999–1005. doi: 10.1016/0026-0495(82)90142-1. [DOI] [PubMed] [Google Scholar]
- Curtius H. C., Zagalak M. J., Baerlocher K., Schaub J., Leimbacher W., Redweik U. In vivo studies of the phenylalanine-4-hydroxylase system in hyperphenylalaninemics and phenylketonurics. Helv Paediatr Acta. 1978 Feb;32(6):461–469. [PubMed] [Google Scholar]
- DALGLIESH C. E., TABECHIAN H. Comparison of the metabolism of uniformly 14C-labelled L-phenylalanine, L-tyrosine and L-tryptophan in the rat. Biochem J. 1956 Apr;62(4):625–633. doi: 10.1042/bj0620625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick P. J., Marshall I. A technique for measuring brain protein synthesis. J Neurochem. 1972 Mar;19(3):577–583. doi: 10.1111/j.1471-4159.1972.tb01375.x. [DOI] [PubMed] [Google Scholar]
- Garlick P. J., McNurlan M. A., Preedy V. R. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J. 1980 Nov 15;192(2):719–723. doi: 10.1042/bj1920719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick P. J., Millward D. J., James W. P. The diurnal response of muscle and liver protein synthesis in vivo in meal-fed rats. Biochem J. 1973 Dec;136(4):935–945. doi: 10.1042/bj1360935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick P. J., Millward D. J., James W. P., Waterlow J. C. The effect of protein deprivation and starvation on the rate of protein synthesis in tissues of the rat. Biochim Biophys Acta. 1975 Nov 18;414(1):71–84. doi: 10.1016/0005-2787(75)90126-4. [DOI] [PubMed] [Google Scholar]
- Golden M. H., Waterlow J. C. Total protein synthesis in elderly people: a comparison of results with [15N]glycine and [14C]leucine. Clin Sci Mol Med. 1977 Sep;53(3):277–288. doi: 10.1042/cs0530277. [DOI] [PubMed] [Google Scholar]
- James W. P., Garlick P. J., Sender P. M., Waterlow J. C. Studies of amino acid and protein metabolism in normal man with L-[U-14C]tyrosine. Clin Sci Mol Med. 1976 Jun;50(6):525–532. doi: 10.1042/cs0500525. [DOI] [PubMed] [Google Scholar]
- Kawamura I., Moldawer L. L., Keenan R. A., Batist G., Bothe A., Jr, Bistrian B. R., Blackburn G. L. Altered amino acid kinetics in rats with progressive tumor growth. Cancer Res. 1982 Mar;42(3):824–829. [PubMed] [Google Scholar]
- Milstien S., Kaufman S. Studies on the phenylalanine hydroxylase system in vivo. An in vivo assay based on the liberation of deuterium or tritium into the body water from ring-labeled L-phenylalanine. J Biol Chem. 1975 Jun 25;250(12):4782–4785. [PubMed] [Google Scholar]
- Moldawer L. L., O'Keefe S. J., Bothe A., Jr, Bistrian B. R., Blackburn G. L. In vivo demonstration of nitrogen-sparing mechanisms for glucose and amino acids in the injured rat. Metabolism. 1980 Feb;29(2):173–180. doi: 10.1016/0026-0495(80)90143-2. [DOI] [PubMed] [Google Scholar]
- Motil K. J., Matthews D. E., Bier D. M., Burke J. F., Munro H. N., Young V. R. Whole-body leucine and lysine metabolism: response to dietary protein intake in young men. Am J Physiol. 1981 Jun;240(6):E712–E721. doi: 10.1152/ajpendo.1981.240.6.E712. [DOI] [PubMed] [Google Scholar]
- Neale R. J., Waterlow J. C. The metabolism of 14C-labelled essential amino acids given by intragastric or intravenous infusion to rats on normal and protein-free diets. Br J Nutr. 1974 Jul;32(1):11–25. doi: 10.1079/bjn19740054. [DOI] [PubMed] [Google Scholar]
- TOLBERT B., WATTS J. H. Phenylalanine requirement of women consuming a minimal tyrosine diet and the sparing effect of tyrosine on the phenylalanine requirement. J Nutr. 1963 May;80:111–116. doi: 10.1093/jn/80.1.111. [DOI] [PubMed] [Google Scholar]
- Trefz F. K., Byrd D. J., Blaskovics M. E., Kochen W., Lutz P. Determination of deuterium-labeled phenylalanine and tyrosine in human plasma with high pressure liquid chromatography and mass spectrometry. Clin Chim Acta. 1976 Dec;73(3):431–438. doi: 10.1016/0009-8981(76)90144-3. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Burke J. F. Effect of burn trauma on glucose turnover, oxidation, and recycling in guinea pigs. Am J Physiol. 1977 Aug;233(2):E80–E85. doi: 10.1152/ajpendo.1977.233.2.E80. [DOI] [PubMed] [Google Scholar]
- Zagalak M. J., Curtius H. C., Leimbacher W., Redweik U. Quantitation of deuterated and non-deuterated phenylalanine and tyrosine in human plasma using the selective ion monitoring method with combined gas chromatography-mass spectrometry. Application to the in vivo measurement of phenylalanine-4-monooxygenase activity. J Chromatogr. 1977 Nov 11;142:523–531. doi: 10.1016/s0021-9673(01)92065-5. [DOI] [PubMed] [Google Scholar]


