Abstract
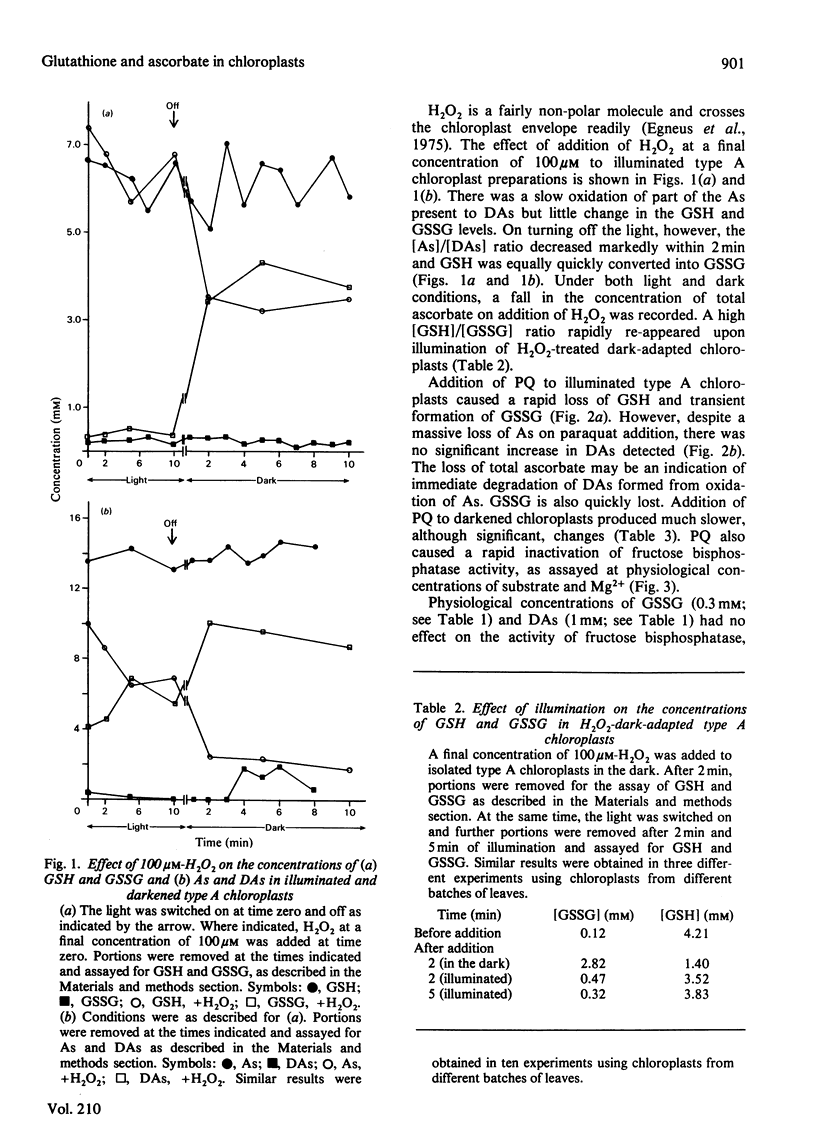
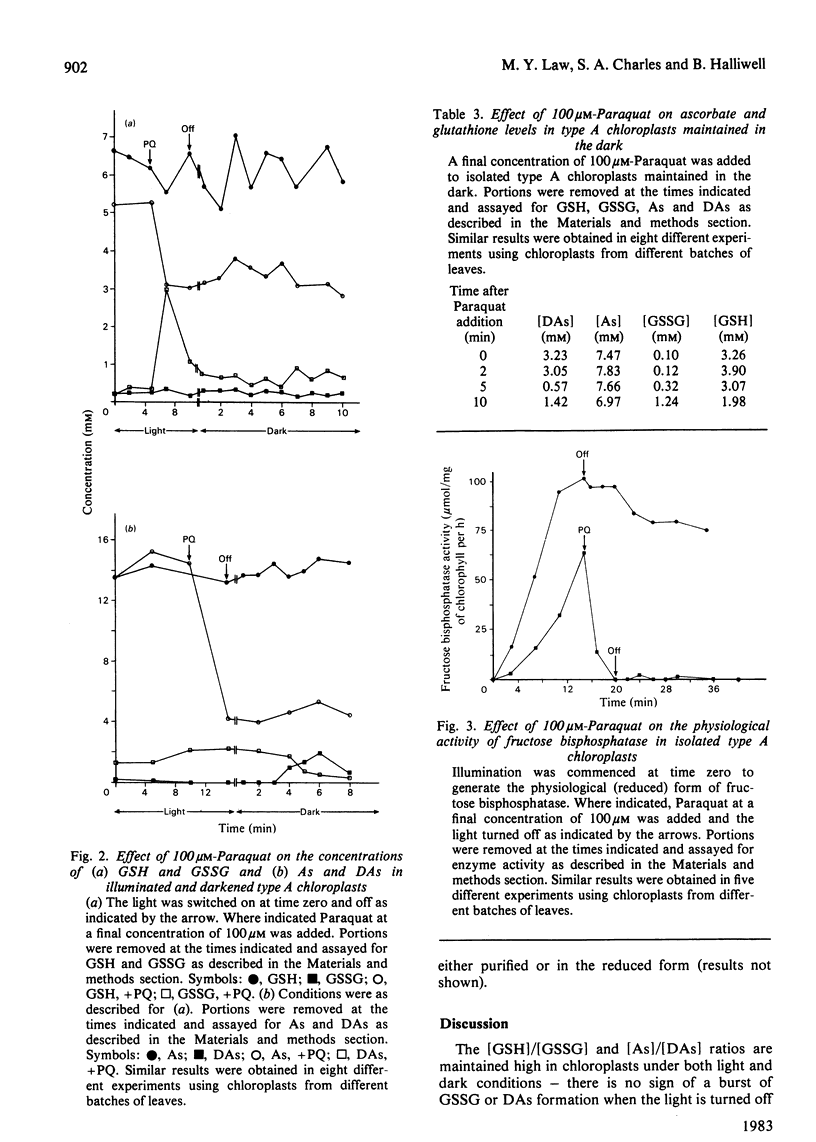
The stroma of spinach chloroplasts contains ascorbic acid and glutathione at millimolar concentrations. [Reduced glutathione]/[oxidized glutathione] and [ascorbate]/[dehydroascorbate] ratios are high under both light and dark conditions and no evidence for a role of oxidized glutathione or dehydroascorbate in the dark-deactivation of fructose bisphosphatase could be obtained. Addition of H2O2 to chloroplasts in the dark decreases the above ratios, an effect that is reversed on illumination. Addition of Paraquat to illuminated chloroplasts caused a rapid oxidation of reduced glutathione and ascorbate, and apparent loss of dehydroascorbate. Paraquat rapidly inactivated fructose bisphosphatase activity, as assayed under physiological conditions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles S. A., Halliwell B. Action of calcium ions on spinach (Spinacia oleracea) chloroplast fructose bisphosphatase and other enzymes of the Calvin cycle. Biochem J. 1980 Jun 15;188(3):775–779. doi: 10.1042/bj1880775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles S. A., Halliwell B. Effect of hydrogen peroxide on spinach (Spinacia oleracea) chloroplast fructose bisphosphatase. Biochem J. 1980 Aug 1;189(2):373–376. doi: 10.1042/bj1890373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles S. A., Halliwell B. Properties of freshly purified and thiol-treated spinach chloroplast fructose bisphosphatase. Biochem J. 1980 Mar 1;185(3):689–693. doi: 10.1042/bj1850689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egneus H., Heber U., Matthiesen U., Kirk M. Reduction of oxygen by the electron transport chain of chloroplasts during assimilation of carbon dioxide. Biochim Biophys Acta. 1975 Dec 11;408(3):252–268. doi: 10.1016/0005-2728(75)90128-0. [DOI] [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Groden D., Beck E. H2O2 destruction by ascorbate-dependent systems from chloroplasts. Biochim Biophys Acta. 1979 Jun 5;546(3):426–435. doi: 10.1016/0005-2728(79)90078-1. [DOI] [PubMed] [Google Scholar]
- Habermann H. M., Handel M. A., McKellar P. Kinetics of chloroplast-mediated photoxidation of diketogluonate. Photochem Photobiol. 1968 Feb;7(2):211–224. doi: 10.1111/j.1751-1097.1968.tb08007.x. [DOI] [PubMed] [Google Scholar]
- Hall D. O. Nomenclature for isolated chloroplasts. Nat New Biol. 1972 Jan 26;235(56):125–126. doi: 10.1038/newbio235125a0. [DOI] [PubMed] [Google Scholar]
- Jablonski P. P., Anderson J. W. Light-dependent reduction of dehydroascorbate by ruptured pea chloroplasts. Plant Physiol. 1981 Jun;67(6):1239–1244. doi: 10.1104/pp.67.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leegood R. C., Walker D. A. Activation of fructose 1,6-bisphosphatase in darkened intact chloroplasts by NADPH. Arch Biochem Biophys. 1981 Dec;212(2):644–650. doi: 10.1016/0003-9861(81)90408-2. [DOI] [PubMed] [Google Scholar]
- Leegood R. C., Walker D. A. Regulation of fructose-1,6-biphosphatase activity in intact chloroplasts. Studies of the mechanism of inactivation. Biochim Biophys Acta. 1980 Dec 3;593(2):362–370. doi: 10.1016/0005-2728(80)90073-0. [DOI] [PubMed] [Google Scholar]
- Okamura M. An improved method for determination of L-ascorbic acid and L-dehydroascorbic acid in blood plasma. Clin Chim Acta. 1980 May 9;103(3):259–268. doi: 10.1016/0009-8981(80)90144-8. [DOI] [PubMed] [Google Scholar]
- Richmond R., Halliwell B. Formation of hydroxyl radicals from the paraquat radical cation, demonstrated by a highly specific gas chromatographic technique. the role of superoxide radical anion, hydrogen peroxide, and glutathione reductase. J Inorg Biochem. 1982 Oct;17(2):95–107. doi: 10.1016/s0162-0134(00)80078-1. [DOI] [PubMed] [Google Scholar]
- Soulié J. M., Buc J., Meunier J. C., Pradel J., Ricard J. Molecular properties of chloroplastic thioredoxin f and the photoregulation of the activity of fructose 1,6-bisphosphatase. Eur J Biochem. 1981 Oct;119(3):497–502. doi: 10.1111/j.1432-1033.1981.tb05635.x. [DOI] [PubMed] [Google Scholar]


