Abstract
Introduction:
Melanoma, a malignant tumor derived from neural crest melanocytes, predominantly affects the skin but can involve any organ with neural crest migration. Metastatic melanoma of unknown origin, particularly when it involves the brain, is associated with significant morbidity, mortality, and a typically poor prognosis.
Case presentation:
The authors present a 71-year-old man with a history of hypertension and seizure disorder who experienced a headache, transient loss of consciousness, and vomiting. Imaging revealed a mass with perilesional edema in the right temporal, right occipital, left frontal, and left periventricular regions. A craniotomy and excision of the right temporal lesion confirmed malignant metastatic melanoma through histological examination. Despite normal findings in skin, mucosal, anogenital, and ophthalmological examinations, and a comprehensive CT scan of the chest, abdomen, and pelvis that revealed no primary tumor, the diagnosis of metastatic melanoma of unknown origin was made. The patient did not receive treatment due to financial constraints.
Clinical discussion:
About 2–6% of melanoma patients present with tumors of unknown primary origin. Brain metastases occur in ~60% of advanced melanoma cases and carry a high risk of spontaneous bleeding. While traditional survival rates are low, surgical resection, stereotactic radiosurgery, immunotherapy, and BRAF/MEK inhibitors can improve outcomes.
Conclusion:
Headaches, lethargy, vomiting, and altered sensorium should prompt investigation for brain metastases from melanoma, even without a detectable primary tumor. Treatment strategies including immunotherapy and stereotactic surgery aim for a median survival of 8–10 months. Socio-economic factors, as highlighted in this case, significantly affect treatment access and patient outcomes.
Keywords: melanoma, metastasis, naevi, unknown primary
Introduction
Highlights
Malignant melanoma with brain metastases and an unknown primary site is a rare and challenging condition. Despite extensive imaging and histopathological evaluations, the primary tumor could not be identified, underscoring the difficulties in determining the source of metastatic melanoma when it is not readily detectable.
The management of melanoma brain metastasis involves surgical resection, radiation therapy as well as immunotherapy and depends upon the size and number of lesions and affected body organs.
Despite existing treatment options, the median survival time remains between 8 and 10 months. The patient’s financial constraints resulted in insufficient follow-up care and restricted access to advanced therapies, adversely impacting his condition and overall prognosis.
Melanoma is a malignant tumor characterized by the malignant transformation of melanocytes derived from neural crest cells1. In about 90% of cases, the skin is the primary organ affected, although it may affect any organ in which the neural crest migrates1,2. Melanoma is the fifth most common cancer diagnosis in the US and it represents 5.6% of all cancer diagnoses3. The natural history of metastatic melanoma with an unknown primary site is not well documented; however, particularly those involving brain metastasis, present significant morbidity and mortality, carrying a poor prognosis. In about 2–6% of patients with melanoma brain metastasis, the primary origin of the tumor cannot be determined4. The treatment for metastatic melanoma to the brain is typically done using surgery and/or radiation therapy. However, advanced-stage melanoma is treated using BRAF-targeted regimens and immunotherapy5.
This is the case report of a 71-year-old male patient with the diagnosis of melanoma with unknown primary origin done after the histopathological analysis of brain lesions in resource-limited settings. This case report provides valuable insights into the diagnosis of melanoma with unknown primary origin, particularly in elderly patients. It underscores the importance of a thorough multidisciplinary approach and highlights the challenges posed by socio-economic constraints. By detailing the clinical presentation, diagnostic process, and treatment decisions, this report serves as a guide to physicians and medical students, enhancing their understanding of melanoma of unknown primary origin and improving patient care strategies.
Case presentation
Clinical history
A 71-year-old male was brought up by his caretaker from a rural primary healthcare center to the emergency department of the tertiary care center in a wheelchair with a severe, dull aching headache for 2 days duration not relieved by over-the-counter analgesics, followed by several episodes of vomiting and blurry vision. Upon further history taking, the headache was acute in onset, initially presenting in the frontal region. After 1 day, the headache became generalized and was more severe in intensity. He had multiple vomiting episodes (>10 times/day) and was non-projectile, non-blood, and bile-stained, containing partially digested food materials. During the management of a patient in the emergency department, he experienced spontaneous loss of consciousness for 2–3 min after which he regained consciousness. He neither had abnormal body movement nor bowel and bladder incontinence during this episode. According to his caretaker, he had a decreased appetite for the past 2 days and had been in a confused state throughout that period. He had a past medical history of hypertension with a treatment regimen of Amlodipine 5 mg once a day for the past 15 years and a seizure disorder for which he takes Levetiracetam 500 mg twice a day for 3 months. He experienced four episodes of seizures over the past three months, with the most recent episode occurring three days ago. The seizures were characterized by sudden loss of body tone, generalized tonic-clonic movements, upward eye rolling, and a return of consciousness within less than a minute followed by post ictal lethargy. His bowel and bladder habits were normal. He had no history of fever, cough, shortness of breath, or abdominal pain. He had no history of malignancy or other tumors, hospital admission and surgery. The patient had not undergone any skin excisions, traumatic mole removals, or cutaneous surgical procedures. After a thorough history and emergency management, the patient was transferred to the Neurosurgery ICU for further evaluation and management.
Physical examination
On physical examination, the patient appeared ill and confused but was oriented to time, place, and person, though he had poor eye contact. When asked for consent for further examination, he seemed hesitant and somewhat unwilling to give his consent. However, after proper counseling, he agreed to the examination.
On examination of his vitals, he had stable vitals except for raised blood pressure (150/100 mmHg). The neurological, respiratory, and cardiovascular examinations were completely normal.
On skin examination, he had thickened dry skin and age spots in the exposed part of the body. A small, ~4×4 mm of hyperpigmented mole was present in the right upper back, and a 2×2 mm mole was present in the medial aspect of his left thigh. However, there was no evidence of asymmetry, thickening, and nodularity of the lesions, or spreading beyond their original boundaries. Additionally, the patient reported no pain, paresthesia, itching, change in size, or bleeding from the hyperpigmented lesions. Apart from the skin findings described earlier, the full examination of the skin was unremarkable. There were no significant findings on the anogenital region except for the multiple skin tags due to chronic anal fissure. Examination of the mucous membrane in the nose and mouth did not reveal any hyperpigmented lesions. Ocular examination, via ophthalmoscopy, revealed bilateral papilledema but did not reveal any foci of melanoma. We were unable to present the photo of the skin lesions as the patient did not provide consent.
No palpable lymphadenopathy was noted throughout the body on general physical examination. He had no family history of cancer.
Laboratory examination
The results of routine serum and urine laboratory investigations notably complete blood count, liver function test, renal function test, arterial blood gas analysis, urine routine examination were all within the normal ranges except for the serum sodium level, which was 132 mg/dl.
Imaging examination
Given his presenting symptoms of severe generalized headache, vomiting, blurred vision, and loss of consciousness, along with a past medical history of multiple seizure episodes, a contrast-enhanced CT scan (CECT) of the head was done to identify any apparent pathology.
Findings in the brain
Multiple hyperdense masses with perilesional edema in the right temporal (Figs. 1A,B), right occipital (Fig. 1E), left frontal (Figs. 1C,D), and left periventricular region (Fig. 1D) with perilesional edema showing enhancement in post-contrast image in Figure 1. Significant mass effect with midline shift was also noted (Fig. 1B). The differential diagnosis included metastatic malignancy and multiple brain abscesses.
Figure 1.
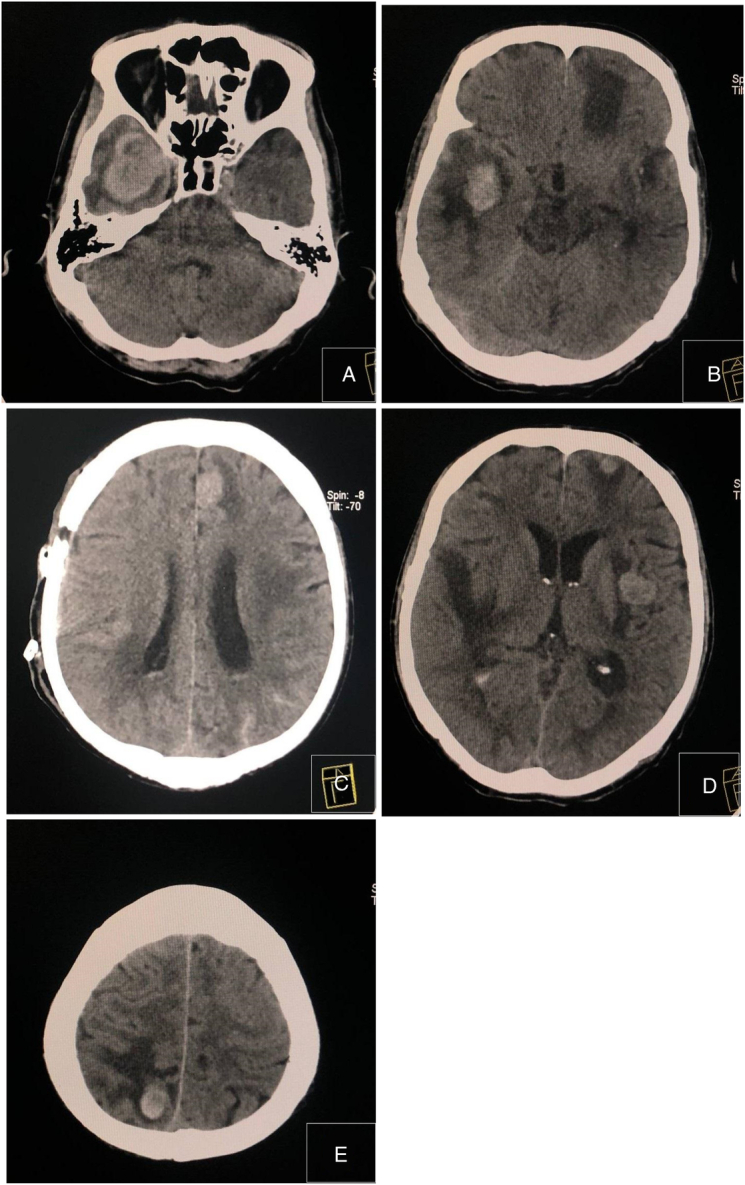
Contrast-enhanced computed tomography scan of the brain. (A–E) Multiple hyperdense masses with perilesional edema in the right temporal, right occipital, left frontal, and left periventricular region with perilesional edema.
A contrast-enhanced CT scan of the brain showing multiple space-occupying lesions with significant perilesional edema, primarily suggests a malignant metastatic tumor to the brain6.
Treatment
The patient was admitted to the neurosurgery intensive care unit due to a deteriorating level of consciousness and Glassgow coma scale (GCS) score, likely caused by increased intracranial pressure as indicated by bilateral papilledema. A multidisciplinary team, including a neuro-physician, oncologist, and neurosurgeons, decided to perform an excisional biopsy of the right temporal mass. This procedure aimed to confirm the tumor’s histology and relieve the intracranial pressure.
Although multiple tumor resections were initially considered, the patient’s overall health, significant mass effect with midline shift, and GCS score was deteriorating, necessitating the excision of a single mass in the right temporal region. Under General Anesthesia, the patient underwent a craniotomy with excision of the right temporal region via an anteromedial approach.
The operation was uncomplicated. Operative findings revealed a firm, rubbery lesion ~5×5 cm from the right temporal region. There was a hematoma present in the tumor bed. Multiple bits of gray-white tissue measuring 3×3×1 cm were sent for histopathological analysis.
Pathological analysis
On histopathological examination, glial tissues were infiltrated by sheets of tumor cells. The tumor cells were discohesive and showed round to polygonal to spindle shape and moderate cytoplasm. Cell borders were well-defined. Nuclei were large, pleomorphic, and showed prominent nucleoli. Some of the cells showed prominent eosinophilic nucleoli. Bizarre cells and multinucleated tumor giant cells were seen. Intra and extracellular melanin pigmentation was noted in most of these tumor cells. Mitosis comprised 3–5/hpf at mitotically active areas. Areas of necrosis, hemorrhage, and blood vessels were noted. Histological features compatible with Malignant metastatic melanoma were noted in Figure 2.
Figure 2.
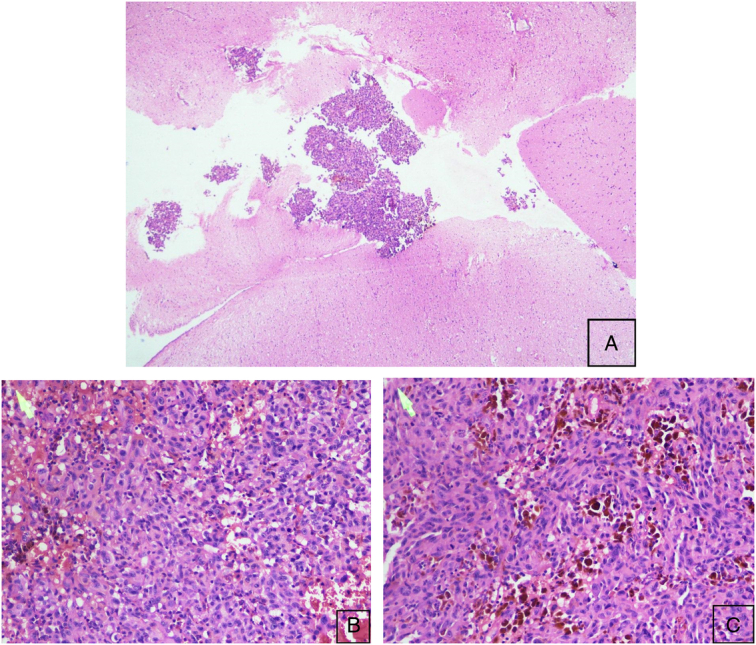
Histopathological examination of malignant metastatic melanoma. (A) Microscopic examination of mass revealing the glial tissues infiltrated by the sheets of tumor cells. (Hematoxylin and eosin, HE 4×). (B, C) Discohesive round to polygonal to spindle-shaped tumor cells. Large pleomorphic nuclei with prominent nucleoli and moderate cytoplasm. Multinucleated giant cells and areas of necrosis, hemorrhages, and blood vessels (Hematoxylin and eosin, HE 20×).
Imaging examination
With the above-mentioned findings in the contrast-enhanced CT scan of the brain and Histopathological analysis, Contrast-enhanced CT scan of chest, abdomen and pelvis was done to identify the presence of primary origin as shown in Figure 3.
Figure 3.
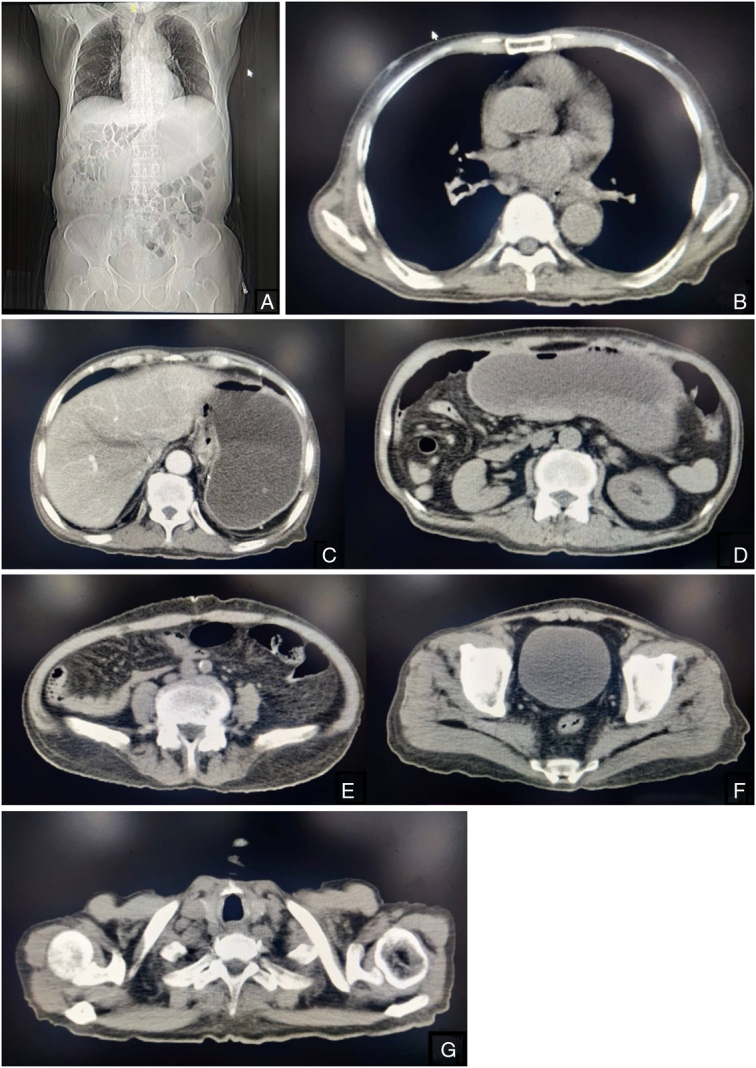
(A–F) Contrast-enhanced computed tomography scan of chest, abdomen and pelvis.
Findings on the contrast CT scan of the chest, abdomen and pelvis demonstrates no evidence of malignant foci or tumors. All examined structures appear normal without any masses, abnormal enhancement, or other signs suggestive of malignancy.
Outcome and follow-up
The patient’s postoperative course was uneventful, with no complications except for a decreased serum sodium level of 126 mg/dl, which was corrected via hypertonic saline infusion. He was extubated on the first postoperative day and transferred to the surgical ward on the third postoperative day. On the seventh postoperative day, he was clinically and hemodynamically stable and was discharged with a prescription for oral steroid for three days, anti-epileptic drugs (Levetiracetam), anti-hypertensive drug (Amlodipine) and oral analgesics (Paracetamol). He was instructed to follow-up after a week with the histopathological reports.
Upon follow-up, the histopathological report confirmed a diagnosis of malignant melanoma. Based on the clinical examination and contrast-enhanced CT scans of the chest, abdomen, and pelvis (Fig. 3), no primary malignancy was identified. Consequently, the diagnosis of metastatic melanoma of unknown origin was done.
The patient’s remaining brain masses in the right occipital, left frontal, and parietal regions were discussed by a multidisciplinary team involving oncologists and neurosurgeons. Given the patient’s advanced age and multiple malignant masses in the brain, the team decided against further surgical intervention. The patient was referred to an oncology center for immunotherapy and stereotactic radiotherapy.
Unfortunately, due to financial constraints and the patient’s refusal to undergo further treatment, he could not follow-up and undergo further recommended treatment. The patient’s caretaker was contacted weekly to monitor his condition, but about a month after the initial surgery, the patient returned to our center with likely neurological complications leading to his death. The most common cause of mortality associated with melanoma brain metastasis is hemorrhage and increased intracranial pressure7. We were unable to determine the exact cause of death due to financial limitations and the caretaker’s refusal to pursue further investigation.
Discussion
Malignant melanoma is a tumor of malignant transformation of melanocytes. Melanocytes are derived from neural crest cells. Though mostly present on the skin at the junction of the dermis and epidermis superficial to the basement membrane, melanocytes can arise in every tissue where neural crest cells migrate1. The characteristic signs of early cutaneous melanoma are recognized by the asymmetry of nevi lesion with an irregular border, non-uniform color with changing shape and size over time, and diameter greater than 6 mm8. Melanoma diagnosis is classified into five stages, 0– IV9. The 5-year survival rate for individuals diagnosed with stage 0 melanoma is 97%, whereas the survival rate is ~10% in those with advanced-stage IV1.
Malignant melanoma is an aggressive malignancy with early dissemination via lymphatic as well as hematogenous routes and has increasing incidence worldwide4. The most common sites of regional melanoma metastasis are the surrounding skin, subcutaneous tissue, and lymph nodes, whereas skin, lung, brain, liver, bone, and intestine are the most common distant sites of melanoma metastasis10. Usually, the tumor has a specific origin in the skin, mucosa, or ocular tissue and is referred to as malignant melanoma of known primary, whereas melanoma with no identifiable primary site is referred to as malignant melanoma of unknown primary4.
Ultraviolet rays exposure via sunlight is the most important risk factor for cutaneous melanoma. Other risk factors include sunburns, the presence of melanocytic or dysplastic naevi, a personal and family history of cutaneous melanoma, phenotypically fair skin, and eyes, with a tendency to freckle, and a high socio-economic status. The pathogenesis of transformation from melanocytes to malignant melanoma involves a sequential genetic model that results in constitutive activation of the oncogenic signal. Activation of BRAF mutation results in benign nevus formation and further progression to melanoma in situ requires a mutation in the telomerase reverse transcriptase (TERT) promoter. The tendency of melanoma to metastasize requires tertiary mutations in cell cycle control genes such as cyclin-dependent kinase inhibitor 2A (CDKN2A) and phosphatase-and-tensin homolog (PTEN) or tumor protein p53 (TP53)11. The most common cause of death in late-stage metastatic melanoma is pulmonary metastasis followed by brain metastasis in about 20–54% of patients8,10.
It’s relatively common for people with advanced-stage melanoma to have brain metastasis. About 60% of melanoma patients end up with brain metastasis as their disease progresses. The propensity of melanoma to metastasize to the brain is not clearly identified but research has suggested several factors such as male gender, a primary tumor on the trunk, and a superficial spreading melanoma9. Most patients with melanoma have a known source for their cancer, but about 2–6% of melanoma patients have a primary tumor that cannot be identified4. The etiology underlying metastatic brain melanoma with an unknown primary origin remains incompletely elucidated. Theoretically, the occurrence of unknown primary melanoma is proposed to stem from primary melanoma lesions that have undergone spontaneous regression, rendering them undetectable. Alternatively, the nevus cells situated ectopically within visceral organs, lymph nodes, or the nervous system may serve as precursors to melanoma metastasis4. Common clinical presentations of melanoma affecting the CNS include headache, neurologic deficits, and/or seizures. Furthermore, melanoma brain metastasis has a high propensity for spontaneous hemorrhage due to early invasion of blood vessels12,13. In our case study, a hematoma was observed in the tumor bed during an excisional biopsy of the right temporal lobe lesion.
Although MRI is more sensitive than CT for detecting brain metastasis, CT remains essential for initial assessment and perioperative management14. In our case study, CT was crucial in identifying the metastatic lesions and guiding urgent surgical intervention. When melanoma is diagnosed in subcutaneous fat, lymph nodes, or visceral organs without an obvious primary source, a thorough evaluation, including ophthalmologic and anogenital exams, is necessary. The recommended work-up for melanoma of unknown primary is controversial, but routine evaluations often involve various imaging modalities such as plain X-ray, CT scan, ultrasound, FDG-PET, whole-body diffusion-weighted imaging (WB-DWI), and MRI9,15,16. 18F FDG-PET/CT is more sensitive (sensitivity: 69%, P <0.001) in detecting primary tumors compared to contrast-enhanced CT or MRI (sensitivity: 41%, P=0.039) in cases of tumors of unknown primary origin17. In our case, we opted for contrast-enhanced CT of the abdomen and pelvis due to patient preference, lack of expertise, patient health status, and financial constraints.
The treatment of melanoma brain metastasis involves a multidisciplinary approach and depends upon the size, number, and location of the lesion as well as other affected body organs. Traditionally, the management of brain metastasis primarily relied on locoregional interventions involving surgical resection and/or radiation therapy. Nonetheless, systemic therapy including immunotherapy and combination therapy with BRAF and MEK inhibitors have demonstrated efficacy within the intracranial compartment, presenting an alternative therapeutic approach for patients with metastatic melanoma18.
Historically, the overall survival after the development of brain metastasis from melanoma has ranged from only 3–6 months19,20. However, patients with solitary or oligometastatic disease who are treated with surgical resection or stereotactic radiosurgery (SRS) have a survival advantage by extending their life expectancy by an additional 3–7 months21. The prognostic factors of brain metastasis depend upon the number of metastatic foci, immunotherapy before melanoma brain metastasis diagnosis, existence of extracranial or leptomeningeal disease, serum lactate dehydrogenase (LDH) levels, and utilization of CNS-directed treatment22.
In addition to the detrimental impact on quality of life, the diagnosis and treatment of brain metastases significantly increase healthcare expenditure, highlighting its substantial socio-economic implications with a monthly expenditure from $7277 to $14 48912,23. It is often observed that individuals from low socio-economic backgrounds have a lower survival rate from melanoma compared to those from higher socio-economic backgrounds24. In our case, these challenges were evident as the patient faced neurological complications and financial constraints, ultimately affecting treatment options and outcomes.
The key message from this case report is the need for policymakers to focus on improving disease management in resource-limited settings. This includes developing better funding and protocols for disease tracking and patient follow-up. Enhanced support and structured approaches can help overcome challenges, ensure better care, and improve outcomes for patients with rare conditions. Additionally, this case report highlights the importance of addressing gaps in healthcare delivery and encourages more effective solutions for managing similar cases in the future.
Limitation
The limitations of this study include the patient’s significant financial barriers, which prevented access to further diagnostic and therapeutic interventions and adversely impacted his care and outcome. Regular monitoring by the caretaker was constrained by financial limitations, leading to inadequate management of the patient’s worsening neurological symptoms. The exact cause of death remained undetermined due to both financial constraints and the caretaker’s refusal to pursue further investigations, highlighting the broader limitations of the healthcare system in resource-constrained settings.
Conclusion
This case report highlights the complexity and challenges associated with diagnosing and managing metastatic melanoma of unknown primary origin, particularly in resource-limited settings. Despite comprehensive diagnostic efforts, including imaging and histopathological analysis in this patient, the primary site remained elusive. His treatment was complicated by financial constraints and the deteriorating clinical condition, which ultimately led to a suboptimal management outcome.
This case report emphasizes several important aspects: the importance of a comprehensive, multidisciplinary strategy for diagnosing and treating melanoma of unknown primary origin, the essential role of imaging in detecting metastases, and the influence of socio-economic factors on the quality of patient care. Advanced-stage melanoma with brain metastases presents significant therapeutic challenges, and while new systemic therapies offer hope, access to these treatments remains uneven, particularly in low-resource settings.
The report advocates for increased awareness and better resource allocation to enhance the management of such cases. Addressing the financial barriers and improving healthcare infrastructure are crucial steps toward improving patient outcomes and quality of care in similar settings. Future efforts should focus on creating more accessible diagnostic and therapeutic options to bridge the gap in care and support for patients with complex cancer diagnoses in resource-constrained environments.
Ethical approval
Not applicable.
Consent
Written informed consent was obtained from the patient’s caretaker for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Source of funding
No such involvement of study sponsors.
Author contribution
K.K.: conceptualization, data curation, writing—original draft, writing—review and editing. B.R.: conceptualization, data curation, writing—original draft, writing—review and editing. A.P.: writing—original draft, writing—review and editing. P.L.S.: writing—original draft, writing—review and editing. S.P.: writing—review and editing. N.P.: data curation. G.S.: supervision. M.B.: supervision.
Conflicts of interest disclosure
The authors declare no conflicts of interest.
Research registration unique identifying number (UIN)
Name of the registry: not applicable.
Unique Identifying number or registration ID: not applicable.
Hyperlink to your specific registration (must be publicly accessible and will be checked) : not applicable.
Guarantor
Kunjan Khanal.
Data availability statement
All the relevant data have been included in the manuscript itself.
Provenance and peer review
Yes
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this articles.
Published online 10 September 2024
Contributor Information
Kunjan Khanal, Email: kunjankhanal22@gmail.com.
Binod Rajbhandari, Email: binodrajbh@gmail.com.
Asim Pandey, Email: pandey.asim09@gmail.com.
Pasang Lamu Sherpa, Email: plsherpa20@gmail.com.
Samriddhi Parajuli, Email: parajulisamriddhi@gmail.com.
Norina Pandey, Email: pnorina581@gmail.com.
Gopal Sedain, Email: newron79@gmail.com.
Maya Bhattachan, Email: mayab@mail.ru.
References
- 1. Heistein JB, Acharya U, Mukkamalla SKR. Malignant Melanoma. 2023 May 22. StatPearls [Internet]. StatPearls Publishing; 2024. [PubMed] [Google Scholar]
- 2. Ali Z, Yousaf N, Larkin J. Melanoma epidemiology, biology and prognosis. EJC Suppl 2013;11:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saginala K, Barsouk A, Aluru JS, et al. Epidemiology of melanoma. Med Sci (Basel) 2021;9:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mremi A, Goodluck G, Sadiq A, et al. Metastatic malignant melanoma of unknown primary site to the brain: a case report. Int J Surg Case Rep 2021;86:106311; Epub 2021 Aug 16. PMID: 34412006; PMCID: PMC8377529. [PubMed]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Proboka G, Tilgase A, Isajevs S, et al. Melanoma unknown primary brain metastasis treatment with ECHO-7 Oncolytic Virus Rigvir: a case report. Front Oncol 2018;8:43; Erratum in: Front Oncol. 2018 May 18;8:172. PMID: 29535971; PMCID: PMC5834433. [PubMed]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smirniotopoulos JG, Jäger HR. Differential Diagnosis of Intracranial Masses. 2020. Diseases of the Brain, Head and Neck, Spine 2020–2023: Diagnostic Imaging [Internet]. Springer; 2020. In: Hodler J, Kubik-Huch RA, von Schulthess GK, ed. Chapter 8. Available from[PubMed]. [PubMed] [Google Scholar]
- 7. Kircher DA, Silvis MR, Cho JH, et al. Melanoma brain metastasis: mechanisms, models, and medicine. Int J Mol Sci 2016;17:1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sundararajan S, Thida AM, Yadlapati S, et al. Metastatic Melanoma. StatPearls [Internet]. StatPearls Publishing; 2024. [PubMed] [Google Scholar]
- 9. Diaz MJ, Mark I, Rodriguez D, et al. Melanoma brain metastases: a systematic review of opportunities for earlier detection, diagnosis, and treatment. Life (Basel) 2023;13:828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Damsky WE, Rosenbaum LE, Bosenberg M. Decoding melanoma metastasis. Cancers (Basel) 2010;3:126–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schadendorf D, Van Akkooi ACJ, Berking C, et al. Melanoma. Lancet 2018;392:971–984. [DOI] [PubMed] [Google Scholar]
- 12. Wilcox JA, DeAngelis LM. Epidemiology and socioeconomic impact of CNS metastases. Springer eBooks; 2020:3–18. [Google Scholar]
- 13. Reddy VU, Suneetha P, Shanthi V, et al. Intracranial hemorrhagic metastases as the first manifestation of an occult melanoma. South Asian J Cancer 2015;4:101–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fink KR, Fink JR. Imaging of brain metastases. Surg Neurol Int 2013;4(Suppl 4):S209–S219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scott JF, Gerstenblith MR. Melanoma of Unknown Primary. Noncutaneous Melanoma [Internet]. Codon Publications; 2018. In: Scott JF, Gerstenblith MR, eds. Chapter 7. Available from:[PubMed]. [PubMed] [Google Scholar]
- 16. Kaleem A, Patel N, Chandra SR, et al. Imaging and laboratory workup for melanoma. Oral Maxillofac Surg Clin North Am 2022;34:235–250. [DOI] [PubMed] [Google Scholar]
- 17. Lee JR, Kim JS, Roh J, et al. Detection of occult primary tumors in patients with cervical metastases of unknown primary tumors: comparison of18F FDG PET/CT with contrast-enhanced CT or CT/MR imaging—prospective study. Radiology 2015;274:764–771. [DOI] [PubMed] [Google Scholar]
- 18. Samlowski W.E., Wu J.K. Management of brain metastases in melanoma UpToDate. https://www.uptodate.com/contents/management-of-brain-metastases-in-melanoma?search=malignant%20melanoma%20metastasis%20brain&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1#H11299788. [Google Scholar]
- 19. Sloot S, Chen YA, Zhao X, et al. Improved survival of patients with melanoma brain metastases in the era of targeted BRAF and immune checkpoint therapies. Cancer 2017;124:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zakrzewski J, Geraghty LN, Rose AE, et al. Clinical variables and primary tumor characteristics predictive of the development of melanoma brain metastases and post-brain metastases survival. Cancer 2011;117:1711–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ramakrishna N, Margolin KA. Multidisciplinary approach to brain metastasis from melanoma; local therapies for central nervous system metastases. Am Soc Clin Oncol Educ Book 2013;33:399–403. [DOI] [PubMed] [Google Scholar]
- 22. Janavicius M, Lachej N, Anglickiene G, et al. Outcomes of treatment for melanoma brain metastases. J Skin Cancer 2020;2020:7520924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vekeman F, Cloutier M, Yermakov S, et al. Economic burden of brain metastases among patients with metastatic melanoma in a USA managed care population. Melanoma Res 2014;24:602–610. [DOI] [PubMed] [Google Scholar]
- 24. Reyes-Ortiz CA, Goodwin JS, Freeman JL, et al. Socioeconomic status and survival in older patients with melanoma. J Am Geriatr Soc 2006;54:1758–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the relevant data have been included in the manuscript itself.


