Abstract
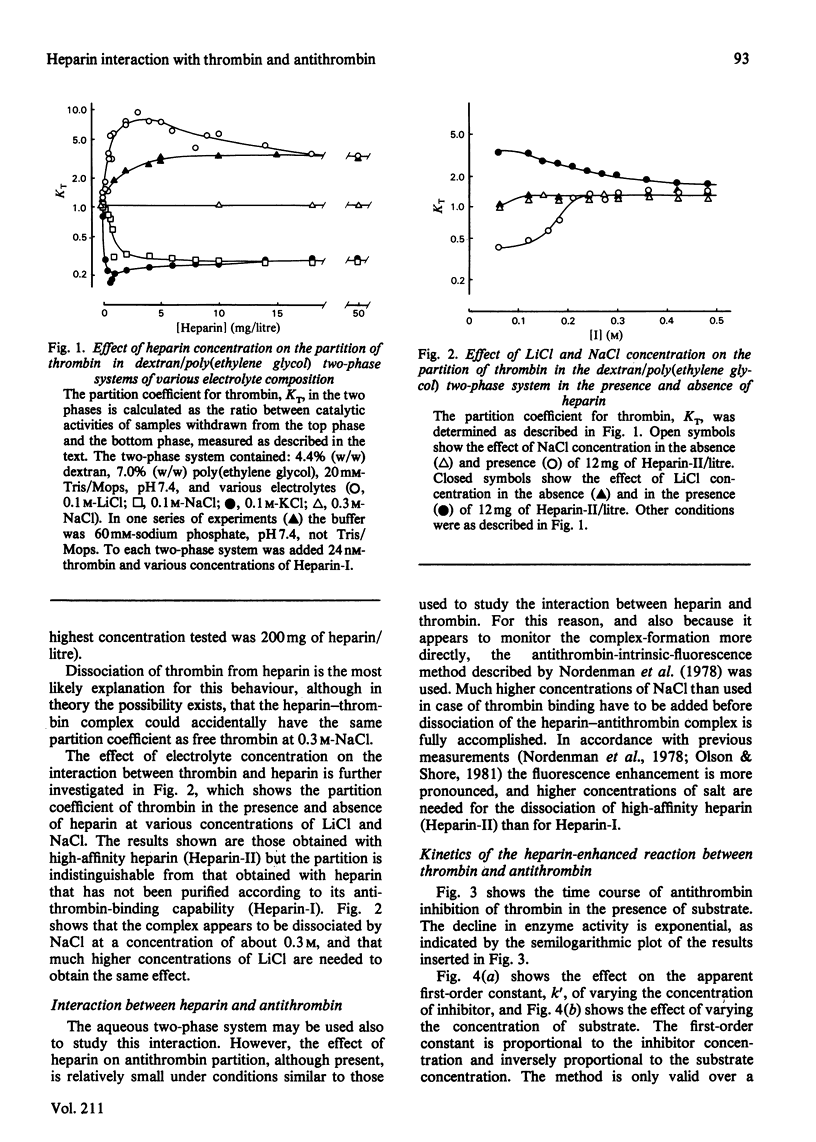
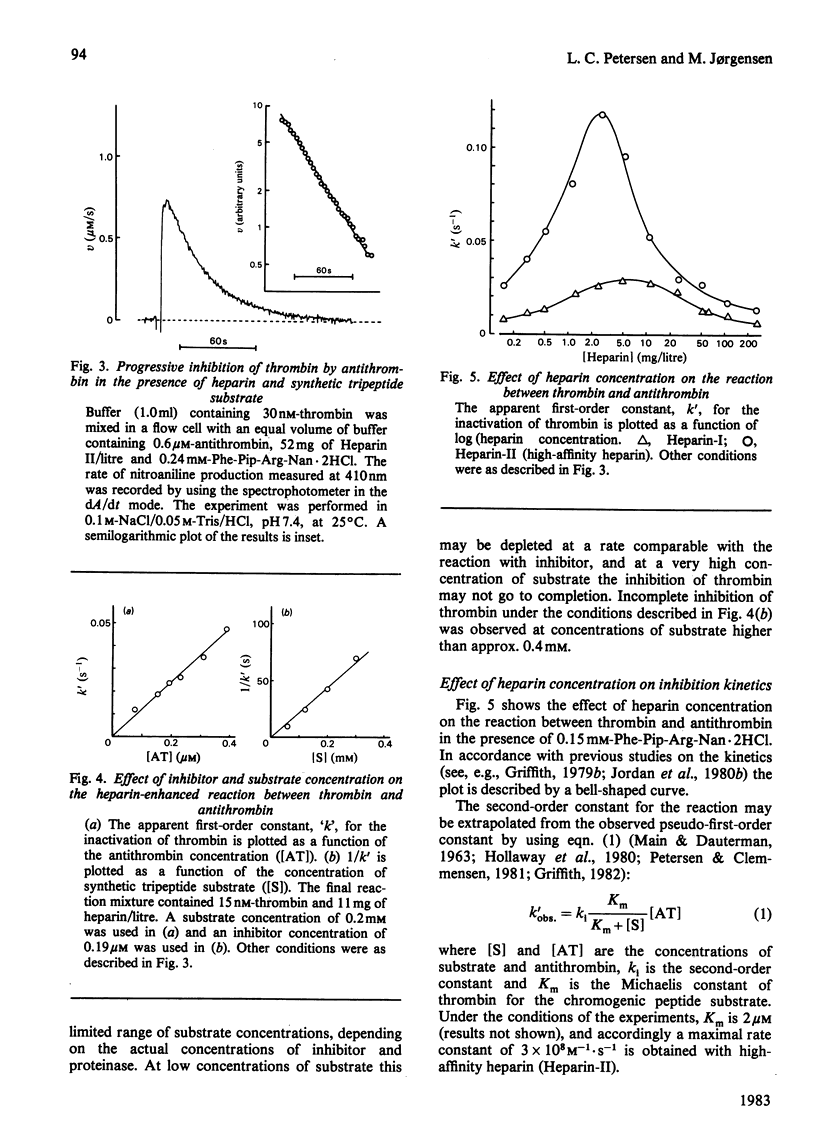
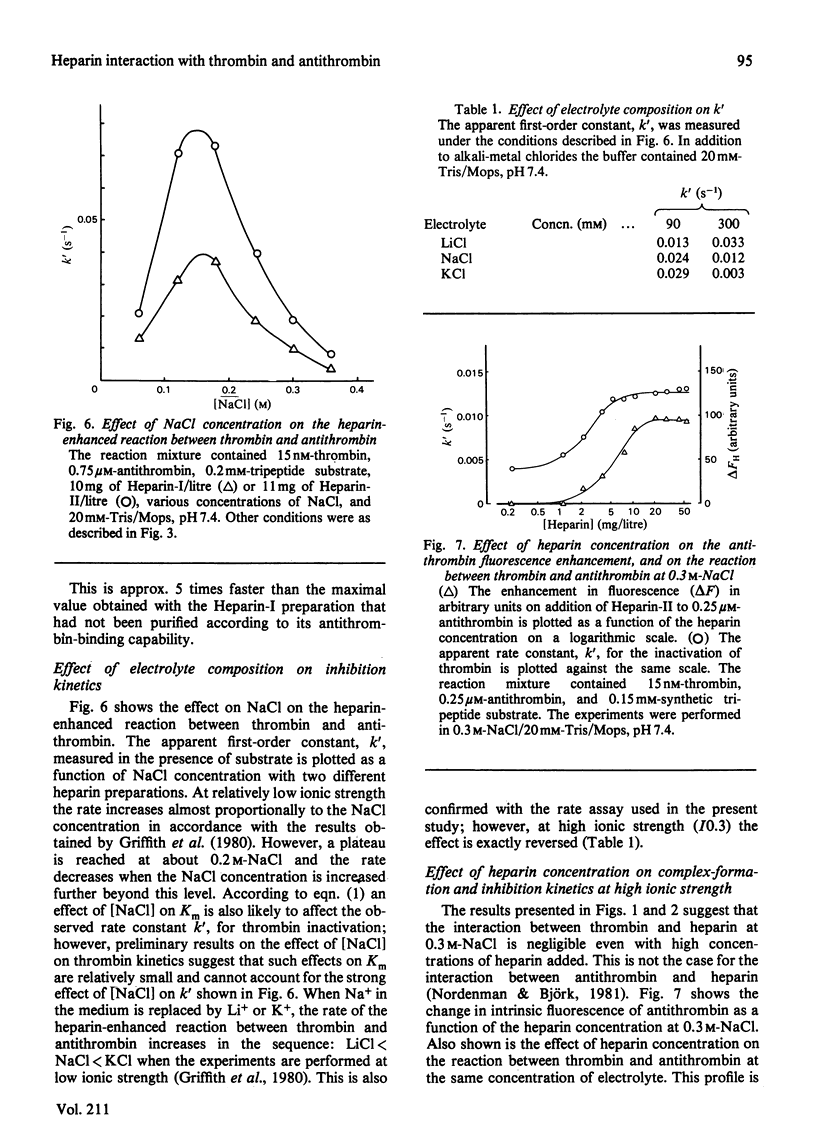
Binding of heparin to thrombin is monitored by means of an aqueous two-phase partition system, and binding of heparin to antithrombin is monitored by means of heparin induced enhancement of the intrinsic fluorescence of the protein. Both types of binding are studied at various electrolyte compositions of the medium. Heparin is displaced from thrombin at lower concentrations of electrolyte than those necessary for its displacement from antithrombin. K+ is more efficient than Na+, which is again more efficient than Li+ in displacing heparin from these proteins. The kinetics of the reaction between thrombin and antithrombin in the presence of heparin were studied by using an assay where synthetic peptide substrate is present in the reaction mixture during the reaction between proteinase and inhibitor. The kinetics are studied at various electrolyte compositions of the medium and the results are compared with those obtained from the binding studies performed under similar conditions. The results are consistent with a model where binding of heparin to antithrombin causes enhancement of the reaction rate, and where this enhancement is abolished again when additional binding of heparin to thrombin takes place on further addition of heparin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chase T., Jr, Shaw E. Comparison of the esterase activities of trypsin, plasmin, and thrombin on guanidinobenzoate esters. Titration of the enzymes. Biochemistry. 1969 May;8(5):2212–2224. doi: 10.1021/bi00833a063. [DOI] [PubMed] [Google Scholar]
- Clemmensen I. Inhibition of urokinase by complex formation with human antithrombin III in absence and presence of heparin. Thromb Haemost. 1978 Jun 30;39(3):616–623. [PubMed] [Google Scholar]
- Griffith M. J., Beavers G., Kingdon H. S., Lundblad R. L. Effect of monovalent cations on the heparin-enhanced antithrombin III/thrombin reaction. Thromb Res. 1980 Jan 1;17(1-2):29–39. doi: 10.1016/0049-3848(80)90291-1. [DOI] [PubMed] [Google Scholar]
- Griffith M. J. Covalent modification of human alpha-thrombin with pyridoxal 5'-phosphate. Effect of phosphopyridoxylation on the interaction of thrombin with heparin. J Biol Chem. 1979 May 10;254(9):3401–3406. [PubMed] [Google Scholar]
- Griffith M. J. Kinetic analysis of the heparin-enhanced antithrombin III/thrombin reaction. Reaction rate enhancement by heparin-thrombin association. J Biol Chem. 1979 Dec 10;254(23):12044–12049. [PubMed] [Google Scholar]
- Griffith M. J. Measurement of the heparin enhanced-antithrombin III/thrombin reaction rate in the presence of synthetic substrate. Thromb Res. 1982 Feb 1;25(3):245–253. doi: 10.1016/0049-3848(82)90244-4. [DOI] [PubMed] [Google Scholar]
- Hollaway M. R., Clarke P. H., Ticho T. Chloroacetone as an active-site-directed inhibitor of the aliphatic amidase from Pseudomonas aeruginosa. Biochem J. 1980 Dec 1;191(3):811–826. doi: 10.1042/bj1910811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C. M., Nemerson Y. Blood coagulation. Annu Rev Biochem. 1980;49:765–811. doi: 10.1146/annurev.bi.49.070180.004001. [DOI] [PubMed] [Google Scholar]
- Jesty J. The kinetics of formation and dissociation of the bovine thrombin.antithrombin III complex. J Biol Chem. 1979 Oct 25;254(20):10044–10050. [PubMed] [Google Scholar]
- Jordan R. E., Oosta G. M., Gardner W. T., Rosenberg R. D. The binding of low molecular weight heparin to hemostatic enzymes. J Biol Chem. 1980 Nov 10;255(21):10073–10080. [PubMed] [Google Scholar]
- Jordan R. E., Oosta G. M., Gardner W. T., Rosenberg R. D. The kinetics of hemostatic enzyme-antithrombin interactions in the presence of low molecular weight heparin. J Biol Chem. 1980 Nov 10;255(21):10081–10090. [PubMed] [Google Scholar]
- Long W. F., Williamson F. B. Potentiation by calcium ions of the antithrombin III inhibition of thrombin. Biochem Biophys Res Commun. 1982 Jan 29;104(2):363–368. doi: 10.1016/0006-291x(82)90645-3. [DOI] [PubMed] [Google Scholar]
- Machovich R., Bauer P. I., Arányi P., Kecskés E., Büki K. G., Horváth I. Kinetic analysis of the heparin-enhanced plasmin--antithrombin III reaction. Apparent catalytic role of heparin. Biochem J. 1981 Dec 1;199(3):521–526. doi: 10.1042/bj1990521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machovich R. Mechanism of action of heparin through thrombin on blood coagulation. Biochim Biophys Acta. 1975 Nov 18;412(1):13–17. doi: 10.1016/0005-2795(75)90334-7. [DOI] [PubMed] [Google Scholar]
- Machovich R., Regoeczi E., Hatton M. W. The influence of heparin, NaCl and CaCl2 on the rate of the thrombin-antithrombin III reaction. Thromb Res. 1979;15(5-6):821–834. doi: 10.1016/0049-3848(79)90191-9. [DOI] [PubMed] [Google Scholar]
- Nordenman B., Björk I. Fractionation of heparin by chromatography on immobilized thrombin. Correlation between the anticoagulant activity of the fractions and their content of heparin with high affinity for antithrombin. 1980 Aug 15-Sep 1Thromb Res. 19(4-5):711–718. doi: 10.1016/0049-3848(80)90044-4. [DOI] [PubMed] [Google Scholar]
- Nordenman B., Björk I. Influence of ionic strength and pH on the interaction between high-affinity heparin and antithrombin. Biochim Biophys Acta. 1981 Feb 5;672(3):227–238. doi: 10.1016/0304-4165(81)90289-0. [DOI] [PubMed] [Google Scholar]
- Nordenman B., Björk I. Purification of thrombin by affinity chromatography on immobilized heparin. Thromb Res. 1977 Dec;11(6):799–808. doi: 10.1016/0049-3848(77)90108-6. [DOI] [PubMed] [Google Scholar]
- Nordenman B., Björk I. Studies on the binding of heparin to prothrombin and thrombin and the effect of heparin-binding on thrombin activity. Thromb Res. 1978 May;12(5):755–765. doi: 10.1016/0049-3848(78)90269-4. [DOI] [PubMed] [Google Scholar]
- Olson S. T., Shore J. D. Binding of high affinity heparin to antithrombin III. Characterization of the protein fluorescence enhancement. J Biol Chem. 1981 Nov 10;256(21):11065–11072. [PubMed] [Google Scholar]
- Petersen L. C., Clemmensen I. Kinetics of plasmin inhibition in the presence of a synthetic tripeptide substrate. The reaction with pancreatic trypsin inhibitor and two forms of alpha 2-plasmin inhibitor. Biochem J. 1981 Oct 1;199(1):121–127. doi: 10.1042/bj1990121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen L. C., Cox R. P. The effect of complex-formation with polyanions on the redox properties of cytochrome c. Biochem J. 1980 Nov 15;192(2):687–693. doi: 10.1042/bj1920687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen L. C. Cytochrome c--cytochrome aa3 complex formation at low ionic strength studied by aqueous two-phase partition. FEBS Lett. 1978 Oct 1;94(1):105–108. doi: 10.1016/0014-5793(78)80916-8. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. D., Damus P. S. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973 Sep 25;248(18):6490–6505. [PubMed] [Google Scholar]
- Wong R. F., Chang T. L., Feinman R. D. Reaction of antithrombin with proteases. Nature of the reaction with trypsin. Biochemistry. 1982 Jan 5;21(1):6–12. doi: 10.1021/bi00530a002. [DOI] [PubMed] [Google Scholar]


