Abstract
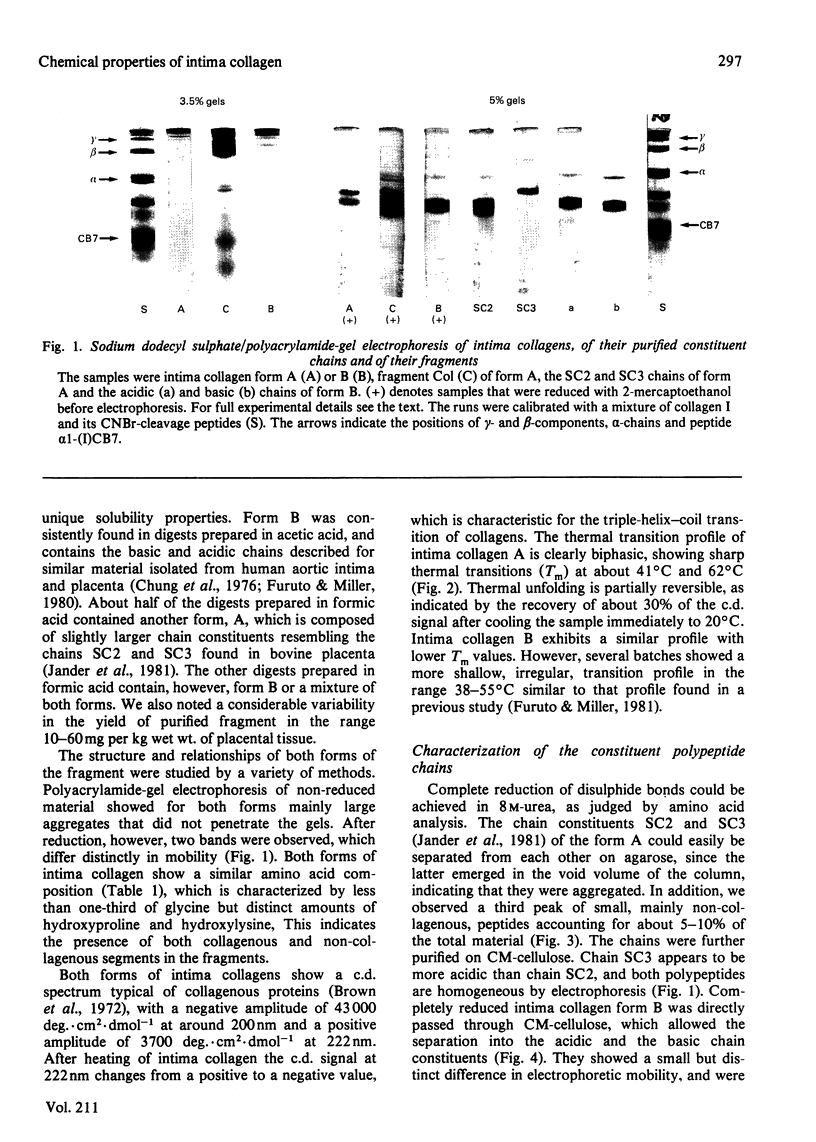
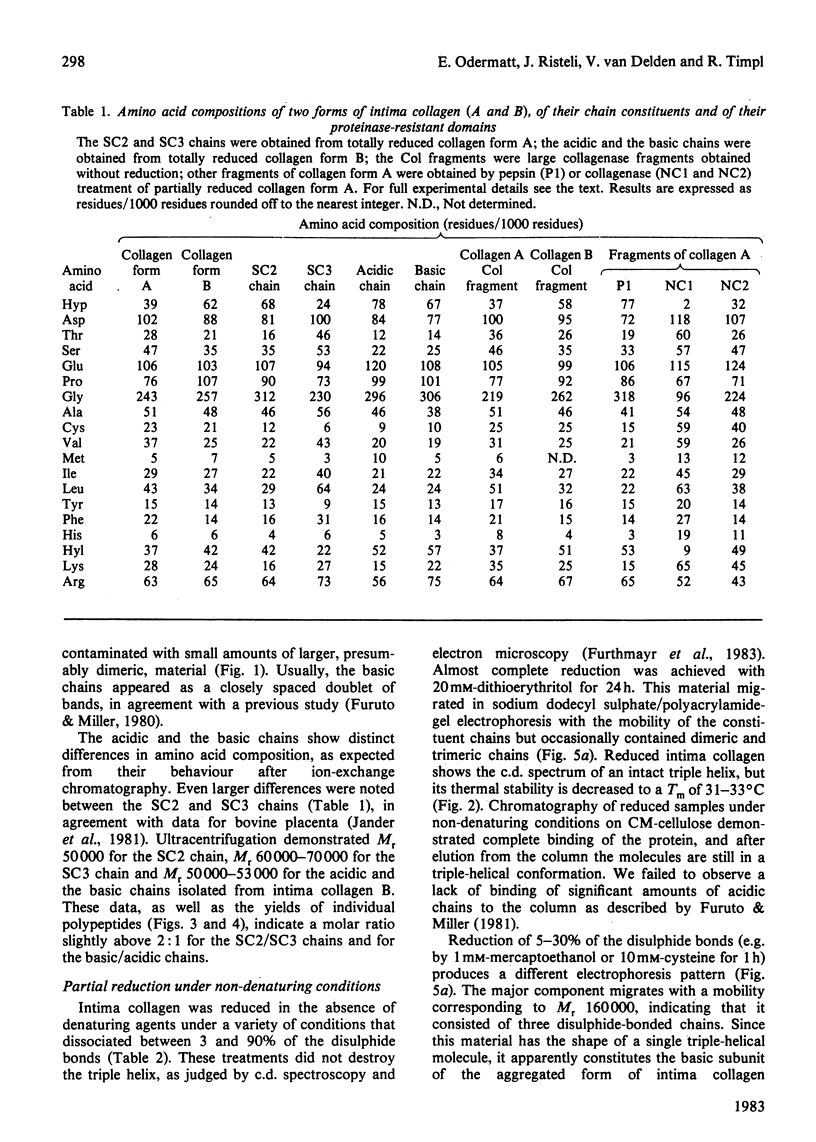
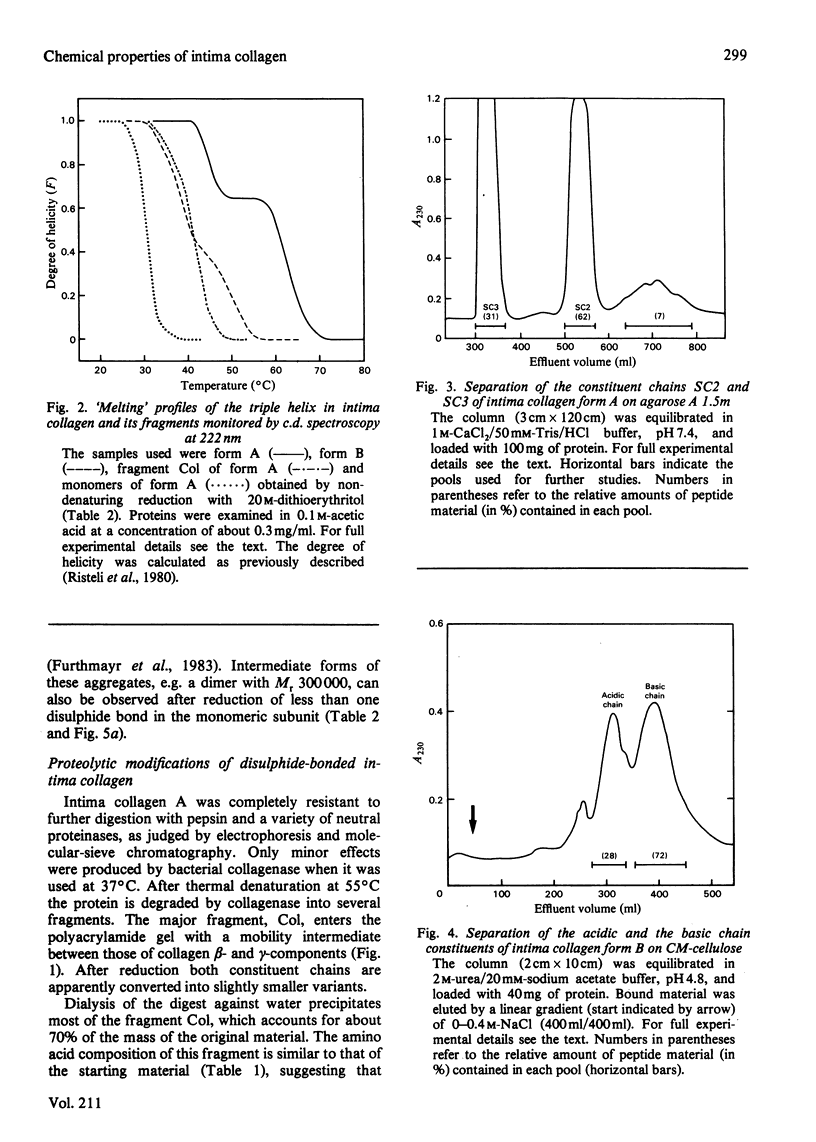
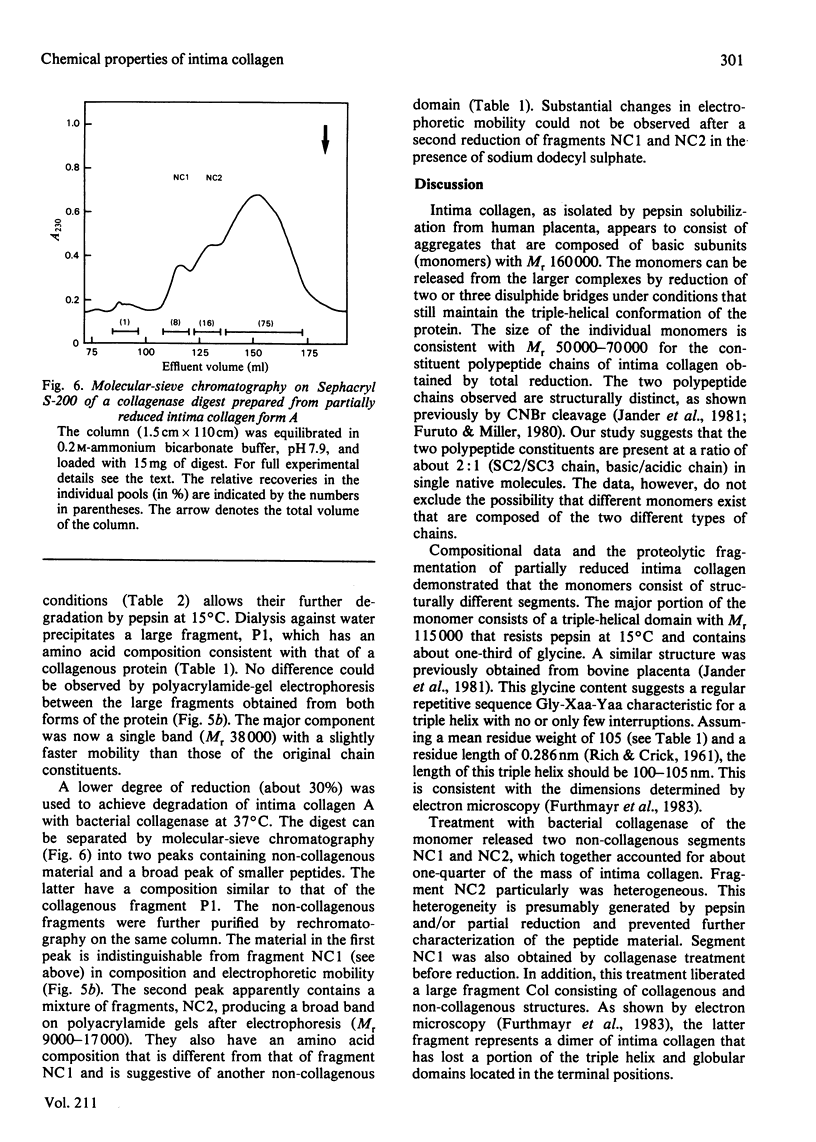
Intima collagen was obtained from pepsin digests of human placenta in two forms, which differ to some extent in the size of their constituent polypeptide chains (Mr 50 000-70 000). These chains are connected by disulphide bonds to large aggregates. The aggregates are arranged in a triple-helical conformation with a remarkably high thermal stability (Tm 41-62 degrees C) and are resistant to further proteolytic digestion. Reduction of as little as 5% of the disulphide bonds produces mainly monomeric triple helices (Mr about 160 000) with Tm 32 degrees C. Partially reduced material can be separated into triple-helical and non-collagenous domains by proteolysis. Pepsin releases a collagenous component with chains of Mr 38 000. Bacterial collagenase liberates two non-collagenous segments (Mr 15 000-30 000) rich in cystine. Treatment with collagenase before reduction separates intima collagen into a large fragment composed of collagenous (Tm 41 degrees C) and non-collagenous structures and a single non-collagenous segment. The data support the electron-microscopical model of intima collagen [Furthmayr, Wiedemann, Timpl, Odermatt & Engel (1983) Biochem. J. 211, 303-311], indicating that the basic unit of the fragment consists of a continuous triple helix joining two globular domains.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bornstein P., Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- Brown F. R., 3rd, Di Corato A., Lorenzi G. P., Blout E. R. Synthesis and structural studies of two collagen analogues: poly (L-prolyl-L-seryl-glycyl) and poly (L-prolyl-L-alanyl-glycyl). J Mol Biol. 1972 Jan 14;63(1):85–99. doi: 10.1016/0022-2836(72)90523-2. [DOI] [PubMed] [Google Scholar]
- Chung E., Rhodes K., Miller E. J. Isolation of three collagenous components of probable basement membrane origin from several tissues. Biochem Biophys Res Commun. 1976 Aug 23;71(4):1167–1174. doi: 10.1016/0006-291x(76)90776-2. [DOI] [PubMed] [Google Scholar]
- Furthmayr H., Timpl R. Characterization of collagen peptides by sodium dodecylsulfate-polyacrylamide electrophoresis. Anal Biochem. 1971 Jun;41(2):510–516. doi: 10.1016/0003-2697(71)90173-4. [DOI] [PubMed] [Google Scholar]
- Furthmayr H., Wiedemann H., Timpl R., Odermatt E., Engel J. Electron-microscopical approach to a structural model of intima collagen. Biochem J. 1983 May 1;211(2):303–311. doi: 10.1042/bj2110303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuto D. K., Miller E. J. Characterization of a unique collagenous fraction from limited pepsin digests of human placental tissue: molecular organization of the native aggregate. Biochemistry. 1981 Mar 17;20(6):1635–1640. doi: 10.1021/bi00509a035. [DOI] [PubMed] [Google Scholar]
- Furuto D. K., Miller E. J. Isolation of a unique collagenous fraction from limited pepsin digests of human placental tissue. Characterization of one of the constituent polypeptide chains. J Biol Chem. 1980 Jan 10;255(1):290–295. [PubMed] [Google Scholar]
- Gibson M. A., Cleary E. G. A collagen-like glycoprotein from elastin-rich tissues. Biochem Biophys Res Commun. 1982 Apr 29;105(4):1288–1295. doi: 10.1016/0006-291x(82)90926-3. [DOI] [PubMed] [Google Scholar]
- Gollwitzer R., Becker U., Timpl R. Isolation and chemical characterization of reduced and aminoethylated polypeptide chains of bovine fibrinogen. FEBS Lett. 1974 Oct 1;47(1):177–180. doi: 10.1016/0014-5793(74)80453-9. [DOI] [PubMed] [Google Scholar]
- Jander R., Rauterberg J., Voss B., von Bassewitz D. B. A cysteine-rich collagenous protein from bovine placenta. Isolation of its constituent polypeptide chains and some properties of the non-denatured protein. Eur J Biochem. 1981;114(1):17–25. [PubMed] [Google Scholar]
- Laurain G., Delvincourt T., Szymanowicz A. G. Isolation of a macromolecular collagenous fraction and AB2 collagen from calf skin. FEBS Lett. 1980 Oct 20;120(1):44–48. doi: 10.1016/0014-5793(80)81042-8. [DOI] [PubMed] [Google Scholar]
- Nowack H., Olsen B. R., Timpl R. Characterization of the amino-terminal segment in type III procollagen. Eur J Biochem. 1976 Nov 1;70(1):205–216. doi: 10.1111/j.1432-1033.1976.tb10971.x. [DOI] [PubMed] [Google Scholar]
- RICH A., CRICK F. H. The molecular structure of collagen. J Mol Biol. 1961 Oct;3:483–506. doi: 10.1016/s0022-2836(61)80016-8. [DOI] [PubMed] [Google Scholar]
- Risteli J., Bächinger H. P., Engel J., Furthmayr H., Timpl R. 7-S collagen: characterization of an unusual basement membrane structure. Eur J Biochem. 1980;108(1):239–250. doi: 10.1111/j.1432-1033.1980.tb04717.x. [DOI] [PubMed] [Google Scholar]
- Timpl R., Bruckner P., Fietzek P. Characterization of pepsin fragments of basement membrane collagen obtained from a mouse tumor. Eur J Biochem. 1979 Apr 2;95(2):255–263. doi: 10.1111/j.1432-1033.1979.tb12961.x. [DOI] [PubMed] [Google Scholar]