Abstract
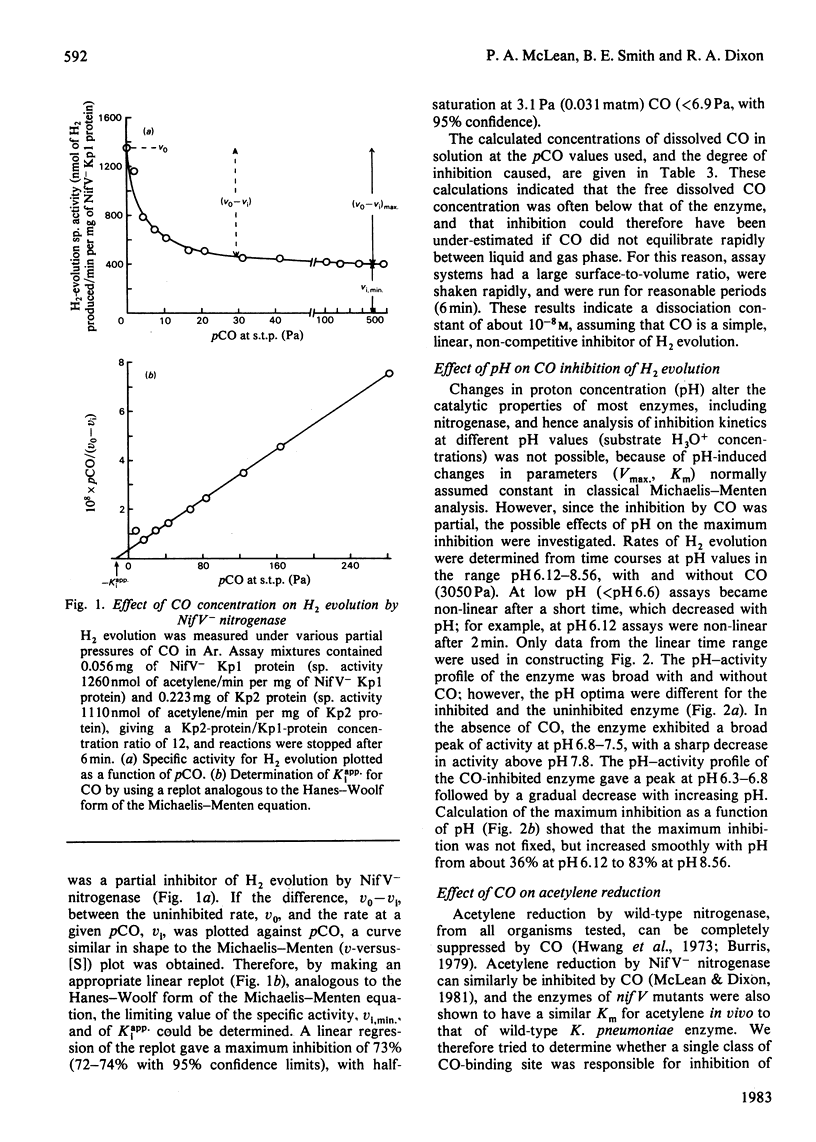
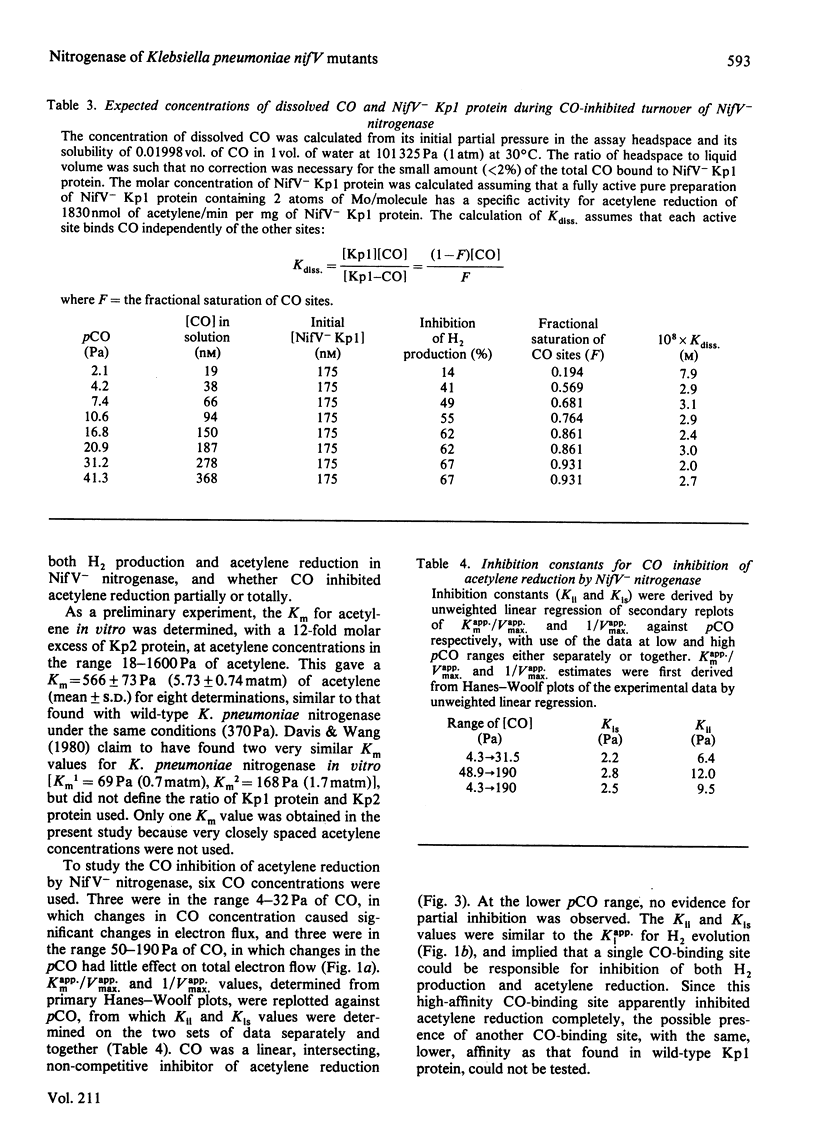
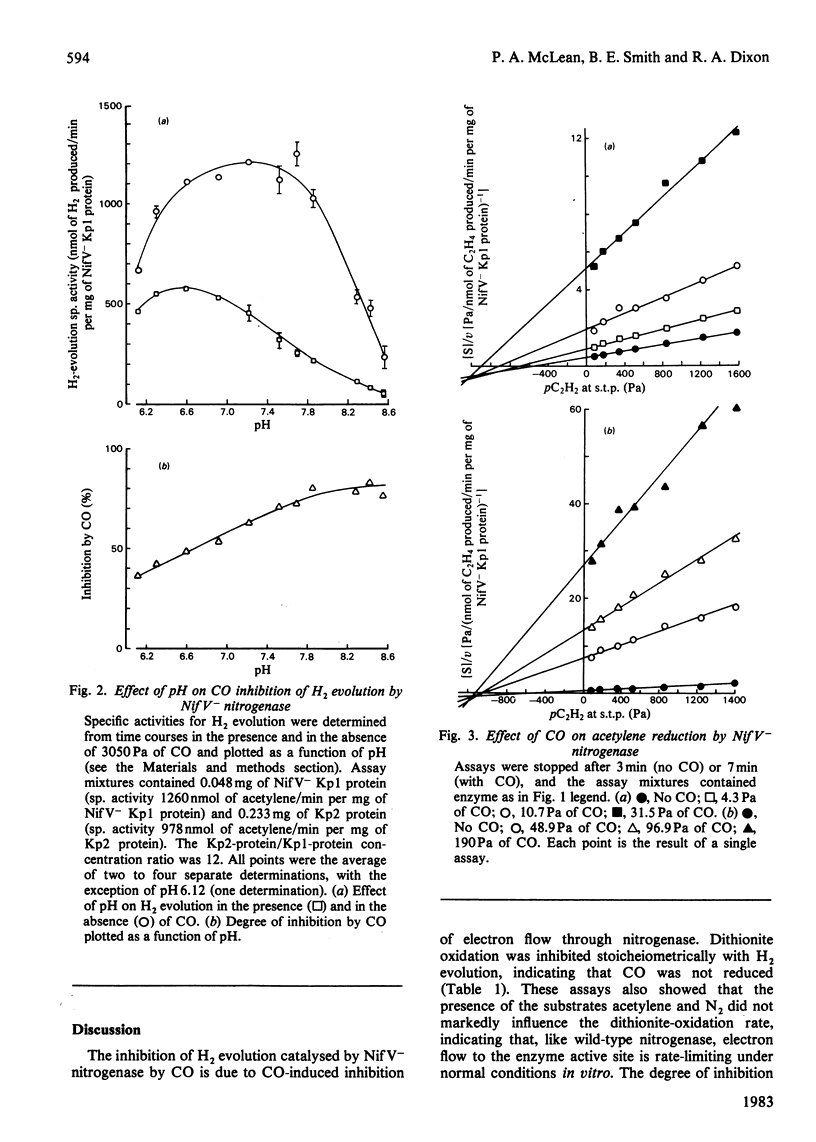
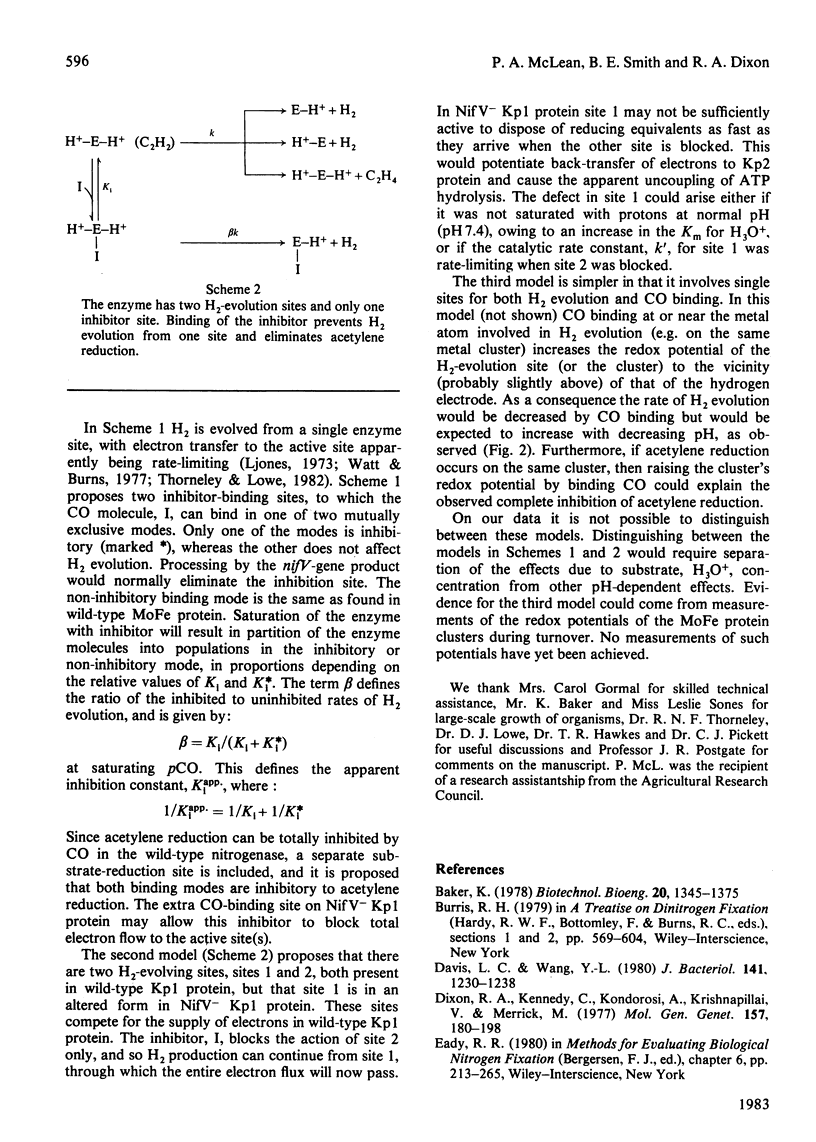
The MoFe protein of nitrogenase from Klebsiella pneumoniae nifV mutants, NifV- Kp1 protein, in combination with the Fe protein from wild-type cells, catalysed CO-sensitive H2 evolution, in contrast with the CO-insensitive reaction catalysed by the wild-type enzyme. The decrease in H2 production was accompanied by a stoicheiometric decrease in dithionite (reductant) utilization, implying that CO was not reduced. However, CO did not affect the rate of phosphate release from ATP. Therefore the ATP/2e ratio increased, indicating futile cycling of electrons between the Fe protein and the MoFe protein. The inhibition of H2 evolution by CO was partial; it increased from 40% at pH6.3 to 82% at pH 8.6. Inhibition at pH7.4 (maximum 73%) was half-maximal at 3.1 Pa (0.031 matm) CO. The pH optimum of the mutant enzyme was lower in the presence of CO. Steady-state kinetic analysis of acetylene reduction indicated that CO was a linear, intersecting, non-competitive inhibitor of acetylene reduction with Kii = 2.5 Pa and Kis = 9.5 Pa. This may indicate that a single high-affinity CO-binding site in the NifV- Kp1 protein can cause both partial inhibition of H2 evolution and total elimination of acetylene reduction. Various models to explain the data are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davis L. C., Wang Y. L. In vivo and in vitro kinetics of nitrogenase. J Bacteriol. 1980 Mar;141(3):1230–1238. doi: 10.1128/jb.141.3.1230-1238.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R., Kennedy C., Kondorosi A., Krishnapillai V., Merrick M. Complementation analysis of Klebsiella pneumoniae mutants defective in nitrogen fixation. Mol Gen Genet. 1977 Nov 29;157(2):189–198. doi: 10.1007/BF00267397. [DOI] [PubMed] [Google Scholar]
- Eady R. R., Smith B. E., Cook K. A., Postgate J. R. Nitrogenase of Klebsiella pneumoniae. Purification and properties of the component proteins. Biochem J. 1972 Jul;128(3):655–675. doi: 10.1042/bj1280655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman R. V., Burris R. H. Electron allocation to alternative substrates of Azotobacter nitrogenase is controlled by the electron flux through dinitrogenase. Biochim Biophys Acta. 1980 Jun 10;591(1):63–75. doi: 10.1016/0005-2728(80)90220-0. [DOI] [PubMed] [Google Scholar]
- Hardy R. W., Knight E., Jr, D'Eustachio A. J. An energy-dependent hydrogen-evolution from dithionite in nitrogen-fixing extracts of Clostridium pasteurianum. Biochem Biophys Res Commun. 1965 Sep 8;20(5):539–544. doi: 10.1016/0006-291x(65)90431-6. [DOI] [PubMed] [Google Scholar]
- Hwang J. C., Chen C. H., Burris R. H. Inhibition of nitrogenase-catalyzed reductions. Biochim Biophys Acta. 1973 Jan 18;292(1):256–270. doi: 10.1016/0005-2728(73)90270-3. [DOI] [PubMed] [Google Scholar]
- Ljones T. Nitrogenase from Clostridium pasteurianum. Changes in optical absorption spectra during electron transfer and effects of ATP, inhibitors and alternative substrates. Biochim Biophys Acta. 1973 Sep 15;321(1):103–113. doi: 10.1016/0005-2744(73)90064-8. [DOI] [PubMed] [Google Scholar]
- McLean P. A., Dixon R. A. Requirement of nifV gene for production of wild-type nitrogenase enzyme in Klebsiella pneumoniae. Nature. 1981 Aug 13;292(5824):655–656. doi: 10.1038/292655a0. [DOI] [PubMed] [Google Scholar]
- Ottolenghi P. The reversible delipidation of a solubilized sodium-plus-potassium ion-dependent adenosine triphosphatase from the salt gland of the spiny dogfish. Biochem J. 1975 Oct;151(1):61–66. doi: 10.1042/bj1510061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Ortiz J. M., Burris R. H. Interactions among substrates and inhibitors of nitrogenase. J Bacteriol. 1975 Aug;123(2):537–545. doi: 10.1128/jb.123.2.537-545.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt G. D., Burns A. Kinetics of dithionite ion utilization and ATP hydrolysis for reactions catalyzed by the nitrogenase complex from Azotobacter vinelandii. Biochemistry. 1977 Jan 25;16(2):264–270. doi: 10.1021/bi00621a017. [DOI] [PubMed] [Google Scholar]


