Abstract
The dUTPase is a key DNA repair enzyme in Mycobacterium tuberculosis, and it may serve as a novel promising anti-tuberculosis target. Stl repressor from Staphylococcus aureus was shown to bind to and inhibit dUTPases from various sources, and its expression in mycobacterial cells interfered with cell growth. To fine-tune and optimize Stl-induced inhibition of mycobacterial dUTPase, we aimed to decipher the molecular details of this interaction. Structural background of the complex between dUTPase and a truncated Stl lacking the repressor C-terminal homodimerization domain has been described, however, the effects of this truncation of Stl on enzyme binding and inhibition are still not known. Using several independent biophysical, structural and enzyme kinetic methods, here we show that lack of the repressor homodimerization domain strongly perturbs both enzyme binding and inhibition. We also investigated the role of a mycobacteria-specific loop in the Stl-interaction. Our results show that removal of this loop leads to a ten-fold increase in the apparent inhibition constant of Stl. We present a high-resolution three-dimensional structure of mycobacterial dUTPase lacking the genus-specific loop for structural insight. Our present data suggest that potent inhibition of mycobacterial dUTPase by Stl requires the wild-type full-length protein context.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-76349-2.
Subject terms: X-ray crystallography, Mass spectrometry, Structure determination, Hydrolases
Introduction
Mycobacterium tuberculosis is the causative agent of tuberculosis (TB), a disease which was responsible for more than 1.3 million deaths in 2022 according to the Global Tuberculosis Report (issued by the World Health Organization)1. The emergence of multidrug-resistant bacteria strains and the co-morbidity of TB and HIV causes an increasing problem in treatment of TB, therefore there is a great need for the development of new anti-TB drugs1,2. Key enzymes in physiologically essential pathways are among the prime targets for such novel anti-TB chemotherapeutics.
The interlinked nucleotide biosynthesis and genome integrity pathways are of crucial importance in all living organisms. Mycobacteria rely on a limited set of pyrimidine nucleotide biosynthesis routes3 and the single pathway towards dTTP biosynthesis necessarily involves the enzyme dUTPase (2’-deoxyuridine 5’-triphosphate nucleotidohydrolase, or DUT). dUTPase plays a central role in regulation of the cellular dUTP/dTTP ratio by catalyzing the hydrolysis of dUTP into dUMP, the substrate of thymidylate synthase. dUTPase also prevents uracil incorporation into DNA. This is of key importance in preventing double-strand breaks and cell death, as increased uracil incorporation into DNA may hyperactivate the base excision repair process, a DNA repair enzyme machinery operating both in prokaryotic and eukaryotic cells3–6. Accordingly, dUTPase is essential in Escherichia coli7, Saccharomyces cerevisiae8 and in Mycobacterium smegmatis9, the laboratory model strain of Mycobacterium tuberculosis. Therefore, inhibition of mycobacterial dUTPase may be a promising way for anti-TB drug development9. Importantly, mycobacterial dUTPases possess a genus-specific insert (sequence alignment provided in Supplementary Figure S1) inducing a surface loop structure that has been shown to be essential for mycobacterial viability making it an important segment in potential drug development9–11.
Stl, the staphylococcal repressor protein (UniProt: Q9F0J8) of staphylococcal pathogenicity islands (SaPIs) was proposed as a universal dUTPase interaction partner, as it is able to interact with multiple enzymes from trimeric and dimeric dUTPase enzyme families12–21. As a consequence of complex formation, the dUTPase activity is inhibited with various efficiency regarding different dUTPase homologues12–16,20. Complete (i.e. 100%) in vitro inhibition of dUTPase activity is only observable upon Ф11 phage dUTPase-Stl complex formation12, an in vivo occurring interaction during Ф11 bacteriophage infection of S. aureus strains containing SaPIs17. It has been shown for several Stl-dUTPase complexes that a tyrosine-rich Stl peptide segment in the N-terminal domain of the Stl protein is inserted into the dUTPase active site, even mimicking some of the dUTPase substrate interactions22. Nevertheless, species-specific differences in Stl-dUTPase binding affinity and inhibition efficiency depend on additional interactions outside the dUTPase substrate binding pocket14,21. The high-resolution crystal structures for Stl-dUTPase complexes invariably contain only a truncated segment of Stl (StlNT) since full-length complexes did not yield diffracting crystals. In this truncated Stl protein, the C-terminal homodimerization domain of Stl is removed20–22. It is not yet described how this truncation of Stl affects its dUTPase inhibition efficiency.
Homotrimeric dUTPases share a high level of structural and residue conservation at their active site, preserving a common enzymatic mechanism23. Nevertheless, dUTPase regions beyond the active site are remarkably different in dUTPases encoded by various species24,25. The mycobacteria-specific surface loop of Mycobacterium tuberculosis DUT (MtDUT) (UniProt: P9WNS5) presents a distinct structural alteration from all other homotrimeric DUTs26. In the absence of these five amino acids long (A133-S137) sequence segment, substrate binding and enzymatic activity is only slightly perturbed (an approximate 10% decrease in MtDUT kcat was observed in vitro), rendering the loop-lacking enzyme variant (MtDUTΔloop) still functional. However, in vivo experiments suggested that the presence of this insert is still essential for the survival of Mycobacterium smegmatis for reasons yet unknown9. The A133-S137 insert is part of the C-terminal arm of MtDUT that forms an integral part of the enzyme active site27 and may also modulate MtDUT-Stl interactions.
In the present study, we aimed to decipher the molecular mechanism of Stl inhibition on MtDUT. Towards this goal we applied structural and functional studies to dissect the contribution of the A133-S137 mycobacteria-specific insert of MtDUT, as well as the Stl C-terminal truncation to the MtDUT–Stl interaction.
Results
The truncated StlNT is a less potent inhibitor of MtDUT
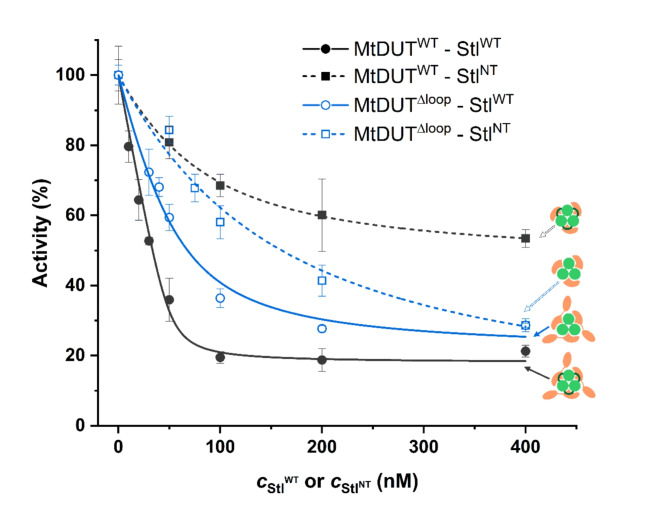
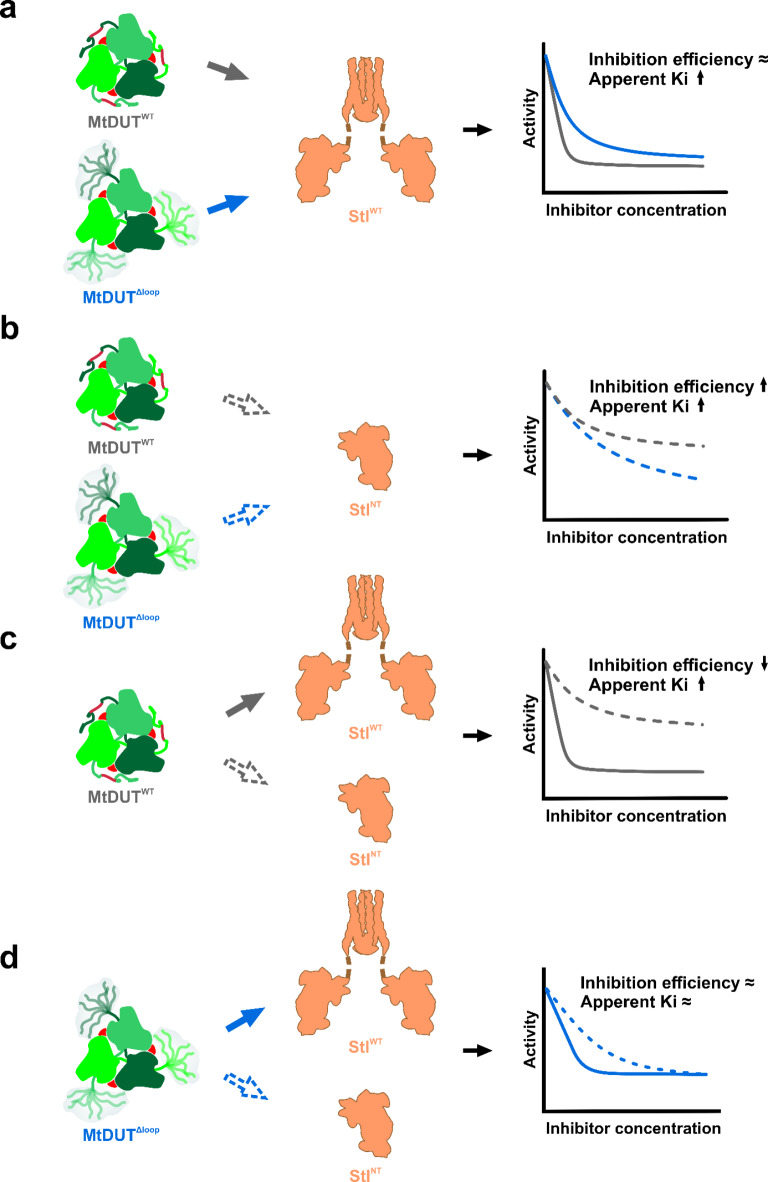
To test the Stl C-terminal domain deletion effect on MtDUT-Stl interaction, we created an StlNT (Stl1–159) construct, similar to the StlN−ter (Stl1–156) previously used in DUT-Stl structural studies20–22, that contains the tyrosine-rich region playing a central role in the interaction14. We also set out to analyse the role of the mycobacteria-specific 133-AGLAS-137 (A133-S137) insert using a previously established MtDUTΔloop loop deletion variant9. We simultaneously investigated the effect of these two missing structural segments on in vitro MtDUT inhibition efficiency using steady-state DUT enzyme activity measurements. Results in Fig. 1; Table 1 show the two key parameters of the inhibition: the inhibition efficiency (at 400 nM Stl concentration) and the apparent inhibition constant Ki, app. Full-length Stl (StlWT) inhibition of MtDUTWT provided (82 ± 3)% inhibition of DUT activity, in accordance with previous data13. In contrast, StlNT displayed a decreased MtDUTWT inhibition with a (47 ± 2)% inhibition efficiency.
Fig. 1.
Activity of MtDUTWTand MtDUTΔloopenzyme variants in presence of varying concentrations of StlWTand StlNT. Quadratic binding equation was fitted to the measured data from which apparent Ki values were determined. The fitted curves are shown in dark grey in case of MtDUTWT and blue in case of MtDUTΔloop. Curves showing MtDUT variants inhibited by Stl are solid lines, while the ones inhibited by StlNT are represented by dashed lines. Symbols representing the different MtDUT-Stl variant complexes consist of a light green dUTPase (with a dark green loop for the wild-type enzyme), StlWT and StlNT are shown in peach colour.
Table 1.
Parameters of MtDUT enzyme activity inhibition by StlWT and StlNT.
| Ki, app (nM) | Inhibition of MtDUT activity*(%) | |
|---|---|---|
| MtDUTWT - StlWT | 2.0 ± 1.2 | 82 ± 3 |
| MtDUTWT – StlNT | 53.2 ± 4.6 | 47 ± 2 |
| MtDUTΔloop - StlWT | 21.1 ± 5.3 | 75 ± 4 |
| MtDUTΔloop – StlNT | 124.3 ± 33.1 | 72 ± 10 |
*At 400 nM inhibitor concentration.
The apparent inhibition constant Ki, app clearly indicated that the most efficient effect is associated with the wild type full-length protein complexes. The 2 nM apparent inhibition constant observed for the StlWT-MtDUTWT complex is at least one order of magnitude higher than in any other combinations. Results therefore suggest that both the removal of the Stl C-terminal homodimerization domain and the genus-specific MtDUT loop negatively interfere with inhibition efficiency. These data suggest considerably weaker interaction with the truncated Stl form (Fig. 1; Table 1).
Absence of the MtDUT loop and truncation of Stl both decrease binding affinity
To complement the kinetic characterization with direct binding assays, we applied biolayer interferometry (BLI). It is important to remark that the KD values of previously published BLI measurements characterising DUT-Stl interactions21,22 did not reflect the true binding affinity, since in these experiments the DUTs were immobilized on the sensor and Stl was in solution. Using this measurement set-up, the dimerization of Stl competes with the binding of its monomeric form to dUTPase. This arrangement interferes with the determination of the correct binding constant for the Stl-DUT complex since Stl concentration comprises both the dimeric and monomeric Stl forms although only the monomeric Stl is capable to bind to dUTPase28. To avoid this complication, we applied a BLI measurement set-up in which the Stl variants (StlWT and StlNT) were immobilized onto the sensor.
The determined KD value for the StlWT–MtDUTWT binding is in the order of picomoles, while the one for StlWT–MtDUTΔloop interaction is an order of magnitude higher. Data indicate that the binding affinity is one order of magnitude weaker if the mycobacteria-specific loop is missing (Table 2).
Table 2.
Complex formation kinetic parameters of StlWT and StlNT with MtDUTWT and MtDUTΔloop.
| Ligand | Analyte | KD* (pM) |
kon (M− 1s− 1) (105) |
koff (s− 1) (10− 5) |
|---|---|---|---|---|
| StlWT | MtDUTWT | < 1 | 2.70 ± 0.01 | < 0.01 ± 0.01 |
| StlWT | MtDUTΔloop | 33 ± 1 | 18.16 ± 0.01 | 5.99 ± 0.14 |
| StlNT | MtDUTWT | 190 ± 1 | 5.42 ± 0.01 | 10.28 ± 0.04 |
| StlNT | MtDUTΔloop | Heterogenous binding | ||
*χ2 value was below 0.3 in all cases.
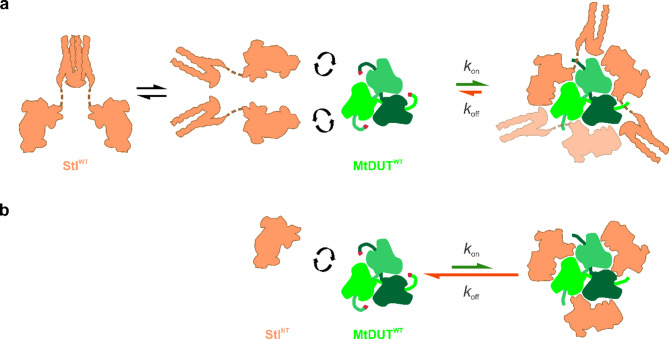
The truncation of Stl into StlNT caused two orders of magnitude increase in the KD value as compared to the StlWT-MtDUTWT complex, arguing for a much weaker interaction. This difference of two orders of magnitude is also observable in case of the measured dissociation rate constants (koff values), but the association rate constants remained in the same order of magnitude (Fig. 6; Table 2, see also Supplementary Table S2 and Supplementary Figures S3–S10).
MtDUT–Stl interaction may lead to different complex stoichiometries
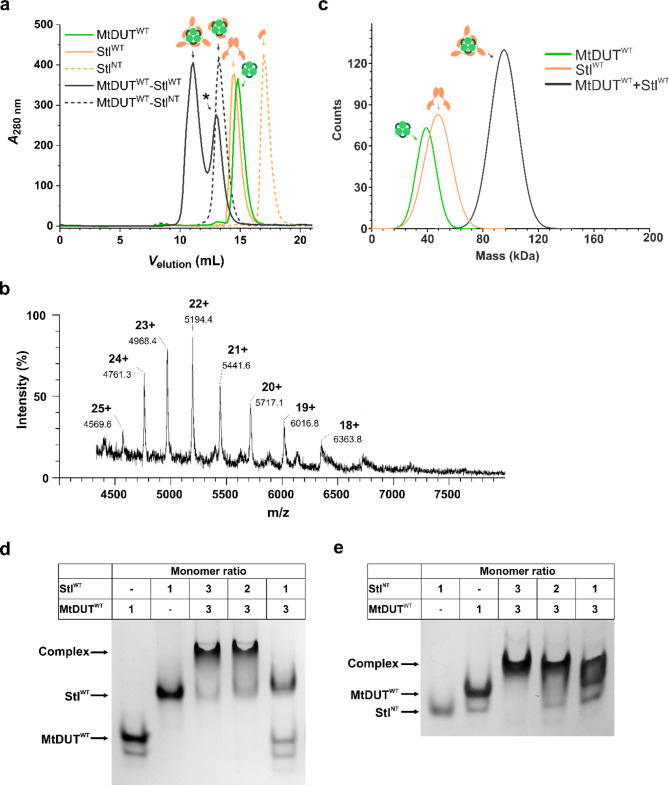
We followed complexation of MtDUT and Stl using size-exclusion chromatography, as well. As shown on Fig. 2a, two peaks are observable on the chromatogram of the MtDUTWT-StlWT complex. The peak elution volumes corresponding to these two peaks (11.1 ml, 13.0 ml) differ from the ones of the individual MtDUTWT (14.8 ml) and StlWT (14.5 ml) (Supplementary Figure S16). It has been shown previously that human dUTPase and StlWT may form two types of complexes: in one case, three Stl molecules are attached to the trimeric dUTPase, whereas in the other case, only two Stl molecules are attached to the trimeric dUTPase14. Taking into account these previous findings, we suggest that the first complex peak in the size exclusion chromatography may correspond to a 3:3 binding stoichiometry, while the second peak, marked with an asterisk, may correspond to a lower, altered stoichiometry. The two types of complexes were only seen with the full-length proteins, and not in the case where StlNT binds to MtDUT (Fig. 2a). We propose that StlNT forms a complex of 3:3 binding stoichiometry with dUTPase, as seen in the crystal structures. We also suggest that steric hindrance may be involved in the formation of the 3:2 complex with full-length Stl and dUTPase, while in lack of the C-terminal Stl domain, the smaller StlNT protein can be better accommodated in the 3:3 complex.
Fig. 2.
In solution complex formation of MtDUTWTwith StlWTor StlNT. (a) Size exclusion chromatography curves showing the chromatography of individual StlWT, StlNT and MtDUTWT and protein complexes. The curves showing chromatography of StlWT and StlNT proteins are presented in peach colour, as solid and dashed lines, with 14.5 ml and 16.9 ml peak elution volumes, respectively. The MtDUTWT curve is shown as a solid light green line with 14.8 ml peak volume. The curves corresponding to the protein-protein complexes are shown as solid and dashed dark gray lines, with 11.1 ml and 13.0 ml for the MtDUTWT–StlWT complex and 13.2 ml elution peak volume for the MtDUTWT–StlNT complex. The symbols showing the individual proteins, and the protein-protein complexes are similar to the ones presented in Fig. 1. (b) ESI-MS spectra of MtDUTWT-StlWT mixture. The peak series of 4569 (with 25 + charge), 4761 (24+), 4968 (23+), 5194 (22+), 5442 (21+), 5717 (20+), 6017 (19+), 6364 (18+) indicate different charged states of a 114.200(± 0.080) kDa particle that corresponds to a complex with 3:2 binding stoichiometry. (c) Mass photometry histograms showing complex formation between MtDUTWT and StlWT or StlNT. The curves and figures corresponding to the individual proteins or the complex are similar to panel (a). (d-e) Native gel electrophoresis showing complex formation between MtDUTWT and StlWT (d), MtDUTWT and StlNT (e) at different mixing ratios.
To further investigate the stoichiometry of the MtDUTWT–StlWT complex, we used native electrospray ionisation mass spectrometry (ESI-MS). A mass of 114.2 kDa was observed consistent with a complex where two StlWT molecules are attached to the trimeric dUTPase (MtDUTWT3StlWT2 complex stoichiometry − 48.4 kDa for MtDUT trimer and 65.8 kDa for two StlWT monomers) (Fig. 2b). Using this method, we did not observe a molecular entity corresponding to the 3:3 stoichiometry.
Since we observed complex formation between MtDUTWT and StlWT with high affinity, it was also of interest to apply mass photometry, another independent method to assess complex formation in a low concentration range, as well. Mass photometry allows to analyse protein–protein interactions in a native-like state in solution, without immobilisation. We have found that a strong complex was formed using a stoichiometric mixture of the interaction partners each in 5 nM concentration (Fig. 2c). Based on the fact that in the mixture of the two proteins, one single complex peak is observed, the KD value is estimated to be in nanomolar range.
In order to augment the present interaction analysis, we performed native gel electrophoresis analysis of MtDUTWT–StlWT and MtDUTWT–StlNT interaction (Fig. 2d, e). The results indicate that a distinct complex band appeared when MtDUTWT and StlWT are mixed at 3:3 and 3:2 ratios, with an additional presence of a small amount of StlWT single component. In contrast, no evident complex band is observed for the MtDUTWT-StlWT 3:1 mixing ratio (Fig. 2d, Supplemetary Fig. S17b).
In case of the MtDUTWT-StlNT interactions the 3:3 mixing ratio yielded a standalone complex band which is accompanied with smaller amounts of single components in the 3:2 sample. The 3:1 mixing also yielded both single component and complex bands (Fig. 2e, Supplementary Fig. S17a). Based on the native PAGE experiments we cannot discern MtDUT-Stl complexes with 3:3 and 3:2 stoichiometry.
Three-dimensional structure of the MtDUT–StlNT complex provides insights into the molecular basis of this interaction
To further explore the stoichiometry and molecular interactions of MtDUT-Stl complex, we performed high-throughput crystallization trials of the MtDUTWT mixed with either StlWT or StlNT with multiple screens and optimization scaffolds. Diffracting crystals were only obtained in the case of the MtDUT-StlNT sample thus, we determined the structure of this complex to 3.4 Å resolution (Supplementary Table S3).
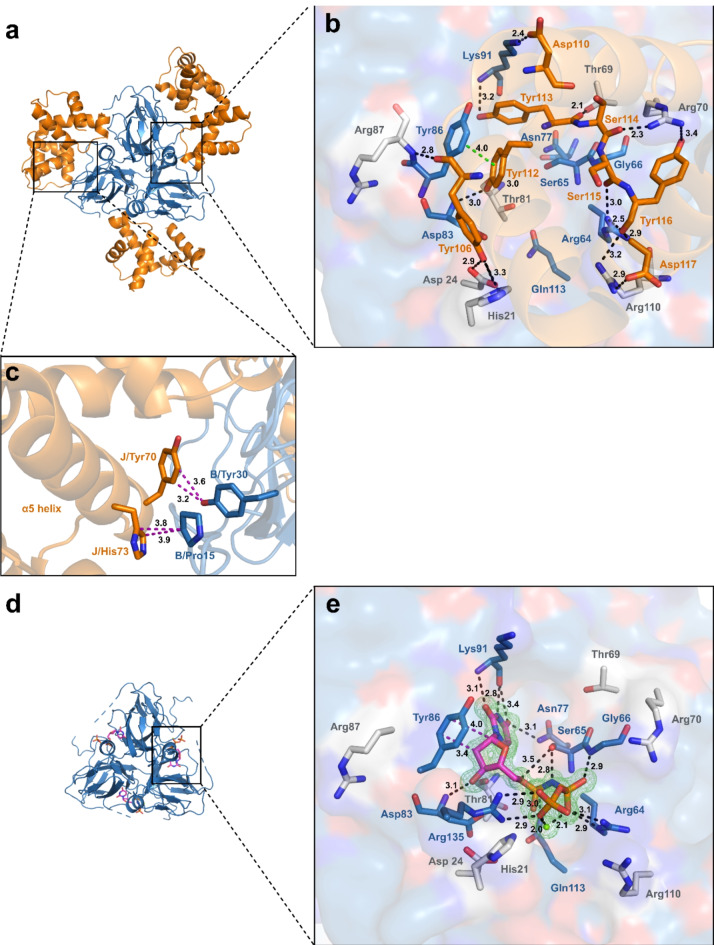
The asymmetric unit of the MtDUTWT-StlNT complex structure (PDB ID:8P8O) is composed of two DUT trimers facing toward each other via the bases of their three-fold symmetry central channel. Similarly to the previously published DUT-StlN−ter structural models20–22, three StlNT monomers are bound to each of the DUT trimers, accounting for a 3:3 MtDUT-StlNT stoichiometry in the crystal (Fig. 3a, Supplementary Figure S11a). The MtDUT-StlNT interaction is fundamentally defined by the protrusion of the StlNT tyrosine-rich segment (Y106-D117) into the MtDUT active site mimicking DUT-substrate interactions, similarly as in20–22 (Fig. 3b). Many MtDUT residues with a critical role in MtDUT-substrate interactions or catalysis26(details in Fig. 3b with blue carbon colour code) are utilized for StlNT binding, likely contributing to MtDUTWT-StlNT complex stability and precluding MtDUT-substrate interactions and enzymatic activity. Specifically, Lys91 residue of MtDUTWT forms hydrogen bonds with both Asp110 and Tyr113 residues of StlNT, which binds to the MtDUT active site pocket recognizing the substrate uracil ring29. MtDUTWT Tyr86, required for dUTP deoxyribose/ribose discrimination29, forms a π-π stacking interaction with Tyr112 of StlNT, the catalytic base Asp83 (MtDUTWT) forms hydrogen bond with the OH group of Tyr112, whereas MtDUTWT Arg64 interacts with Ser115 and Tyr116 of StlNT. Moreover, additional residues outside the MtDUT active site provide hydrogen-bond and polar interactions to StlNT tyrosine-rich segment (Fig. 3b with grey carbon color code for MtDUT residues; and Supplementary Table S4). StlNT interaction additionally induces conformational changes to the MtDUT N-terminal Ser18-Asp22 segment in the vicinity of the active site, which provides a prominent negatively charged surface region of MtDUT (Supplementary Fig. S11). Intriguingly, the mycobacteria-specific AGLAS loop of MtDUT is only partially resolved in the MtDUT-StlNT structure, whereas no electron density is present corresponding to the MtDUT C-terminal arm that folds over the DUT active site and has a decisive role in enzyme catalysis30,31. Since StlNT has shown different inhibition kinetics on MtDUTWT compared to the inhibition of MtDUTΔloop (cf. Fig. 1), the possibility has raised that the loop itself may play a steric role in C-terminal arm movements of MtDUTWT.
Fig. 3.
Structure-based comparison of the MtDUTWT-StlNTand MtDUTΔloop–dUPNPP complexes. (a) Overall structure of MtDUTWT–StlNT complex. MtDUTWT is represented as blue cartoon, StlNT is represented as orange cartoon. (b) MtDUTWT active site interactions with StlNT. Substrate analogue interacting residues are displayed as blue stick, while residues interacting only with StlNT are represented as grey sticks, StlNT interacting residues are shown as orange sticks. Interactions are indicated as dashed black (polar), π- π stacking interaction is shown as dashed green line (with distances measured in Å). (c) Interactions of StlNT α5 helix with MtDUTWT. Interacting residues are shown as sticks. Colouring of the proteins are the same as in panel (a). Interactions are shown as dashed purple lines (with distances measured in Å). (d) Overall structure of MtDUTΔloop in complex with dUPNPP substrate analogue. MtDUTΔloop is displayed as blue cartoon, substrate analogue is represented as sticks. (e) Active site interactions of MtDUTΔloop in complex with dUPNPP. MtDUTΔloop is represented as surface. The representation of interacting residues is the same as on panel (b). The omit map around substrate analogue and Mg2+-ion (green sphere) is contoured in green isomesh at 3.0σ level. Interactions are indicated as dashed black (polar) and purple (hydrophobic) lines (with distances measured in Å). Individual panels were created using PyMOL 2.5.4 (Schrodinger, LLC; https://www.pymol.org/). The figure was assembled using CorelDRAW 2020 (Corel Corporation; https://www.coreldraw.com).
The MtDUTWT–StlNT complex structure (PDB ID:8P8O) shares notable similarities to a recently published MtDUTWT-StlN−ter structure (PDB ID: 7PWX)21, including the Stl-DUT 3:3 molecular arrangement and DUT: Stl interactions detailed above. Nevertheless, the MtDUTWT–StlNT complex is crystallized in a different space group (P212121) compared to MtDUTWT-StlN−ter (P21), and accordingly, it also provides dissimilar structural features. In contrast to the MtDUT-StlN−ter structure, in the presented structure, the α5 helix of each StlNT monomer is found in an ordered conformation and is modelled in the electron density map. Within, His73 and Tyr70 from StlNT are packed against Pro15 and Tyr30 from MtDUT providing aromatic-proline and polar interactions, respectively, that supplement the MtDUT-StlNT complex interface in one of the MtDUT-StlNT subunit pairs (Fig. 3c).
Stl C-terminal domain (StlCT) is not present in the crystallized StlNT construct, nevertheless it modulates MtDUT-Stl interactions in solution studies (Figs. 1 and 2). To interrogate its potential role in complex formation, we generated multiple AlphaFold models including three copies of both MtDUTWT and StlWT. The MtDUTWT–StlWT Alphafold complex structures reproduce the overall 3:3 complex assembly found in the MtDUTWT-StlNT crystal structure with the MtDUTWT C-terminal arm dislodged by Stl. The MtDUTWT C-term arm is found in a disordered ensemble with no molecular contacts with either Stl or MtDUT protein core (Supplementary Fig. S13). Intriguingly, the StlCT domains provide an additional trimer oligomerization surface, in all generated models, albeit the conformation of this additional assembly varies across the models. AlphaFold models of a MtDUTΔloop-StlWT 3:3 complex are showing similar results (Supplementary Fig. S14).
The mycobacteria-specific surface loop of MtDUTWT contributes to the stabilization of the enzyme C-terminal arm
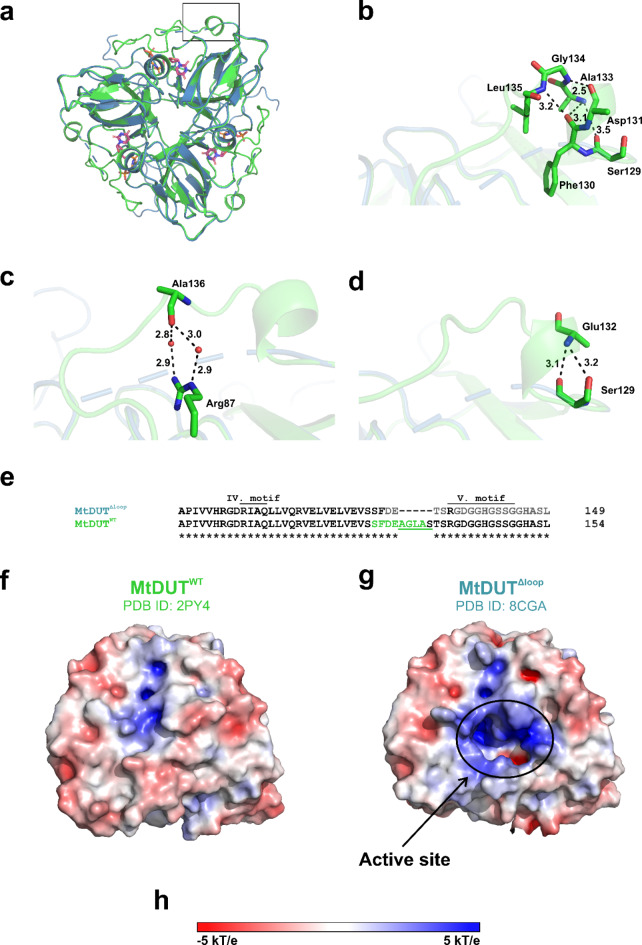
To investigate how the mycobacteria-specific loop contributes to the MtDUT-Stl interaction, we additionally crystallized MtDUTΔloop together with the dUPNPP substrate analogue and determined their complex structure to 1.3 Å resolution (Supplementary Table S3). The MtDUTΔloop structure (PDB ID:8CGA) contains a single protomer in its asymmetric unit, that, together with its symmetry-related copies builds up an all-β jellyroll fold trimeric DUT assembly prototypical for MtDUT and trimeric dUTPases23,32 (Fig. 3d). The MtDUTΔloop structure shares a high degree of overall similarity with MtDUTWT (PDB ID: 2PY4) based on a root mean square deviation (RMSD) of 0.20 Å for 378 Cα atoms of the MtDUT trimers (Fig. 4a). The MtDUTΔloop structure features conserved interactions between dUPNPP and the enzyme core that are described for the MtDUTWT-dUPNPP structure30. Namely, Lys91 and Asn77 residues form hydrogen bonds with the uracil ring of dUPNPP, Tyr86 forms van der Waals interactions with the deoxyribose, and Asp83 interacts with the 3’-OH of the deoxyribose with a hydrogen bond. The Mg2+-chelated triphosphate moiety of substrate analogue is coordinated by Arg64, Ser65, Gly66, Gln113, and Arg135 (equivalent to Arg140 in MtDUTWT) residues (Fig. 3e). In the MtDUTΔloop structure, the C-terminal arm is mostly disordered, in contrast to its ordered conformation when it is folded over the active site in MtDUTWT (PDB ID: 2PY4). The last resolved residue of the polypeptide chain is Phe130, and beyond, only Arg135 from the 5th conserved motif could be modelled into the electron density, likely given its key contribution to nucleotide substrate binding and catalysis33 (Figs. 3e and 4e). This indicates that the C-terminal arm is rendered more flexible in the absence of the mycobacteria-specific loop. Particularly, several polar interactions, H-bonds, and salt bridges that contribute to the stabilization of the C-terminal arm over the active site in MtDUTWT (PDB ID: 2PY4), are abolished in MtDUTΔloop structure, due to the deletion of key participating residues and accompanying conformational changes (Fig. 4b, c, d). The absence of an ordered C-terminal arm remarkably changes the electrostatic surface of MtDUTΔloop compared to MtDUTWT. The mostly positively charged active site and its surroundings become exposed in MtDUTΔloop, as it is not shielded by the C-terminal arm (Fig. 4e, f).
Fig. 4.
Comparison of the MtDUTΔloop and MtDUTWT structures. (a) Comparison of overall crystal structures of MtDUTWT and MtDUTΔloop. The wild-type enzyme is represented as light green cartoon, the surface loop lacking enzyme is shown as blue cartoon. The dUPNPP substrate analogue is shown as sticks. (b, c) Interaction network of amino acid residues forming the A133-S137 loop responsible for anchoring C-terminal arm. Interacting residues are shown as stick. Hydrogen bonds, salt bridges are shown as dashed black lines (with distances measured in Å). (d) Further anchoring interactions of the C-terminal arm that are resolved only in MtDUTWT crystal structure. Interacting residues are shown as sticks, hydrogen bonds are represented as dashed black lines (with distances measured in Å). (e) Sequence alignment comparing the C-terminal segments of MtDUTΔloop and MtDUTWT. The amino acid residues not resolved in the MtDUTΔloop structure are shown in grey, the residues highlighted in (b–d) panels are show in green. The AGLAS surface loop region is underlined in green. (f-g) Electrostatic potential of the molecular surfaces of MtDUTWT (f) and MtDUTΔloop(g) excluding the resolved region of the His6 epitope tag and the Met1 residue, to show a comparable structural region as MtDUTWT. The position of the active site is highlighted with a circled area. (h) The colouring of the electrostatic surface potential scale. Individual panels (a–h) were created using PyMOL 2.5.4 (Schrodinger, LLC; https://www.pymol.org/), panel (e) was created and the figure was assembled using CorelDRAW 2020 (Corel Corporation; https://www.coreldraw.com).
To assess the conformation of the MtDUT C-terminal arm in the MtDUTΔloop structure, we compared AlphaFold models of MtDUTΔloop and MtDUTWT. An ensemble of 10 MtDUTWT models unanimously show that the C-terminal arm adopts a closed state (Supplementary Fig. S12a), similarly as seen in the MtDUTWT-dUPNPP structure (PDB ID: 2PY4). In the MtDUTΔloop models the C-terminal arm is found with either in a closed state or in multiple different outward oriented poses, underscoring its apparent flexibility (Supplementary Fig. S12b).
Discussion
Our present kinetic and interaction analysis investigations of MtDUT-Stl complexes clearly indicate that the full-length Stl is a tightly binding, highly efficient inhibitor of MtDUT, whereas truncation of either protein negatively interferes with complexation (Figs. 5 and 6). We show that StlCT, the homodimerization domain of Stl22 plays an important role in the complex formation and inhibition of the dUTPase enzymatic activity. The significant role of this homodimerization domain in the dUTPase complexation is a novel finding. It is also underlying the key importance of the fact that dissociation of the StlWT homodimer is essential for dUTPase binding and is also interfering with the Stl repressor function12,28,34. We also established that the mycobacteria-specific loop of MtDUT may modulate Stl complexation and MtDUT C-terminal arm conformation. As this segment is dissimilar from the human host dUTPase architecture, its structural consideration may assist the design of MtDUT-targeted anti-TB drugs.
Fig. 5.
The effect of different truncations on inhibitory potential of StlWTand StlNTexerted on MtDUTWTand MtDUTΔloop. (a–d) Schematic figures showing the different effects of Stl and MtDUT truncation on MtDUT activity. The steady-state activity curves are represented similarly as on Fig. 1. The representation of the molecules is the same as on Figure S2. Conclusions drawn regarding the inhibition efficiency and apparent inhibition constant (Ki) are related to MtDUTWT (on panel a, b) or to Stl (on panel c, d). Figure was created using CorelDRAW 2020 (Corel Corporation; https://www.coreldraw.com).
Fig. 6.
Schematic representation of enzyme-inhibitor protein complex formation for the different truncated mutants. (a) MtDUTWT-StlWT complex formation. (b) MtDUTWT–StlNT complex formation. (a, b) The representations of the individual molecules are similar to the ones as on Figure S2. The protein-protein complexes are shown as the complexes of the individual proteins, which representations are based on MtDUTWT-StlNT structural model (PDB ID: 8P8O), the exact conformation of the proteins and their binding stoichiometry are not taken into account. The magnitude of kon and koff values determined in the BLI measurements are represented as green and red arrows, respectively. Please note, that the dimensions of kon and koff values are different. Therefore, only the same kind of rate constants from the different complex formation reactions are comparable with each other. Figure was created using CorelDRAW 2020 (Corel Corporation; https://www.coreldraw.com).
Examining the structural function of the MtDUT A133-S137 mycobacteria-specific insert, we observed that its deletion renders the majority of the C-terminal arm disordered in the MtDUTΔloop crystal structure, suggesting an increased flexibility of this segment. Compared with MtDUTWT, we concluded that this loop plays an important role in anchoring the enzyme C-terminal arm above the active site (Fig. 4b and c). In agreement with this, an earlier study investigating the MtDUT C-terminal arm movements using random acceleration molecular dynamics (RAMD) method revealed that residues 130–137 (including this insert region), and 149–154 stay fixed to the enzyme core during the whole catalytic cycle, while the intercepted region (residues 138–148) comprising residues Arg140 and His145 shows much higher flexibility during repeated RAMD runs35.
The inhibition efficiency of MtDUT activity using StlWT or StlNT as inhibitors, was only marginally altered by deletion of the A133-S137 insert in MtDUTΔloop. Comparing the inhibition efficiency in the case of MtDUTWT–StlNT and MtDUTΔloop–StlNT reveals a limited inhibition efficiency for MtDUTΔloop, which could be consistent with its increased arm flexibility that hinders effective StlNT interaction and inhibition.
The effect of deletion of the A133-S137 loop on MtDUT-Stl binding affinity is not fully conclusive based on our experiments. When StlWT is loaded on the sensor in the BLI experiments, the rate constant of complex dissociation is increased by several orders of magnitude in the case of the MtDUTΔloop-StlWT complex, while the association rate constant shows a much smaller increase. These kinetic effects result in a weakened complexation, that may be related to the increased MtDUT C- terminal arm flexibility in MtDUTΔloop. However, when either MtDUTWT or MtDUTΔloop is loaded onto the sensor, the binding affinity is not significantly affected. This somewhat unclear effect of the absence of the A133-S137 loop is also due to the fact that in the case of interaction between MtDUT and StlNT, heterogeneous binding events prevent quantitative evaluation of the interaction affinity.
Theoretically, it seemed plausible that the shorter StlNT construct containing the tyrosine-rich region may inhibit MtDUT activity more effectively than StlWT, since the binding of a shorter protein could be more facilitated. On the contrary, we found that the truncation of StlWT to StlNT did not result in a more effective MtDUT inhibitor. In the crystal structure of MtDUTWT-StlNT complex, each active site of the enzyme is blocked by a StlNT. Nevertheless, the strength of enzyme-inhibitor interaction was considerably reduced when StlNT was used. Thus, the C-terminal Stl domain (StlCT) has a direct or indirect effect in the formation of a stronger interaction, resulting in a more efficient inhibition of MtDUTWT activity. One possible explanation for this phenomenon is suggested by the AlphaFold models of MtDUTWT3:StlWT3 complex, which show that an additional oligomerization surface is formed between C-terminal domains of StlWT monomers (Supplementary Fig. S13). This could provide an avidity effect, which may contribute to the formation of a stronger complex compared to MtDUTWT3:StlNT3 complex. AlphaFold models for the MtDUTΔloop3:Stl3 complex also show the potential avidity effect, however to a lesser extent (cf. Supplementary Fig. S14).
The C-terminal arm of homotrimeric DUTs is a flexible structural element whose active site interactions are critical for dUTP substrate binding and hydrolysis27,36–38. For both hDUT and MtDUT, it was suggested that there is a low probability of complete opening of the arm during substrate binding and product release, but it is more likely that several slightly altered conformations that are partially closed over the active site are adopted by the enzyme during catalysis35,36.
Upon complex formation with Stl N-terminal domain constructs, the C-terminal arm of DUTs is displaced from the active site as seen in crystal structures of MtDUT-StlNT(PDB ID: 8P8O), MtDUT-StlN−ter (PDB ID: 7PWX)21, Φ11 DUT-StlN−ter (PDB ID: 6H4C)22, hDUT-StlN−ter (PDB ID: 7PWJ)21 and lvDUT-StlN−ter (PDB ID: 7DLV)20 complexes. All these structures display a trimer DUT state where all three active sites are occupied by the Stl N-terminal domain, thus this state is not compatible with DUT activity. In contradiction to this expectation, in vitro kinetic studies with numerous DUTs from different origins - except for the Φ11 phage DUT – invariably show 20–100% remnant enzyme activity in the presence of Stl12–16,20. One explanation for this observation might be that the C-terminal arm re-occupies the DUT active site to aid substrate hydrolysis. However, given the slow dissociation rate of Stl-DUT complexes and their tight interaction affinity12, this scenario is not supported by the experimental data. An alternative explanation may suggest that not all of the active sites can be simultaneously occupied by Stl in solution. Accordingly, our gel filtration and ESI-MS studies of MtDUT-StlWT complex formation propose more than one binding stoichiometry, namely 3:3 and 3:2 MtDUT-StlWT complex stoichiometry. Small-angle X-ray scattering measurements of the hDUT–Stl complex also suggested the parallel presence of 3:3 and 3:2 DUT-Stl stoichiometry in solution14. Since full inhibition of MtDUT enzymatic activity requires all of its active sites to be blocked by the inhibitor, the potential presence of a complex with 3:2 binding stoichiometry will always result in remaining enzymatic activity.
Conclusions
Our present study creates the full and relevant framework for understanding the actual extent of inhibition that can be achieved in the interspecies DUT-Stl complexes. We wish to emphasize that using the full-length, wild-type proteins is essential in providing relevant information for the design of potent MtDUT inhibitors since the truncation of Stl, found to be essential to form a crystallizable protein complex, unfortunately led to a loss of crucial information about effective inhibition characteristics. In addition to performing experiments in the full-length Stl protein context, we also introduced an optimized experimental setup for the determination of strength of DUT-Stl complex formation, based on a relevant recent study from our laboratory28. In our present study, the binding affinity of the Stl: dUTPase complex has been determined in a correct experimental set-up using biolayer interferometry, and the data indicate the formation of an exceptionally strong complex of dUTPase and its protein inhibitor (KD in the picomolar range). Due to the essential character of dUTPase, this robust complexation forms a promising basis for further inhibitor design. However, our results indicate that complete MtDUT inhibition is not achievable, neither by StlWT nor by StlNT. Further targeted engineering of Stl will be necessary to obtain Stl-based inhibitors that can eliminate mycobacterial DUT activity in vivo.
Materials and methods
Protein sequence comparison
The sequence comparison of several mycobacterial, other bacterial (based on sequence alignment in9 and protein-protein BLAST search using https://blast.ncbi.nlm.nih.gov/Blast.cgi webserver), human and two Staphylococcal phage dUTPase sequences was carried out using Clustal Omega (https://www.ebi.ac.uk/jdispatcher/msa/clustalo). Afterwards, the multiple sequence alignment was fine-tuned manually.
Cloning and mutagenesis
Plasmid encoding StlNT Stl (UniProt: Q9F0J8) deletion mutant (Stl1–159) was generated from Stl-encoding pGEX-4T-1 vector (GE Healthcare) by introducing a stop codon within the Stl sequence with site directed mutagenesis using partially overlapping Stl1-159_FW forward and Stl1-159_Rev reverse primers. MtDUTWT (UniProt: P9WNS5) and MtDUTΔloop were amplified from pET-15b plasmid (Merck KGaA) using Avi-MtDUT_FW forward, Avi-MtDUT_Rev reverse primers and cloned to pAN4 vector (Avidity, LLC) in frame with Avi-tag, between XhoI and KpnI cleavage sites.
The Avi-tagged Stl constructs were created from Stl-encoding pGEX-4T-1 plasmid with the insertion of Avi-tag sequence on C-terminal end of GST-fused Stl sequence using Stl-Avi-mut-F-fin and Stl-Avi-mut-R-fin forward and reverse primers. Then GST-Stl-Avi was amplified using GST-Stl-Avi-pan4-F and GST-Stl-Avi-pan4-R primers and inserted into pAN4 vector between KpnI and XhoI restriction sites. The StlNT-Avi encoding gene was generated by mutagenesis of GST-Stl-Avi-encoding pGEX-4T-1 plasmid, afterwards cloned to pAN4 vector using GST-Stl-Avi-pan4-F and GST-Stl-Avi-pan4-R primers, resulting in the translation of a thrombin cleavable GST-tagged StlNT-Avi construct. The oligonucleotide primers used are listed in Supplementary Table 1.
Protein expression and purification
Proteins in this study were expressed in E. coli BL21 (DE3) Rosetta cells (Novagen, Merck KGaA) in LB media, induced by 0.5 mM Isopropyl β-D-1-thiogalactopyranoside after OD600 reached 0.5–0.6. The wild-type (WT), and Δloop mutant MtDUT enzymes were expressed from pET-15b vector for 4 h, at 37 °C. The genes encoding full-length Stl (StlWT) and StlNT proteins were expressed from pGEX-4T-1 plasmid for 4 h, at 30 °C. The cells were harvested by centrifugation for 30 min at 4 °C at 4000 rpm, then resuspended in precooled PBS and centrifuged for 30 min at 4 °C at 4000 rpm. The pellets were frozen and stored at -80 °C. The MtDUT and the Stl protein variants were purified similarly as previously described13,26.
The MtDUT variant containing cell pellets were resuspended in 50 ml of lysis buffer (300 mM NaCl, 50 mM TRIS pH 8.0, 0.5 mM EDTA, 10 mM 2-mercaptoethanol, 1 mM PMSF, 5 mM benzamidine, 6.4 mM lysozyme and a cOmplete ULTRA EDTA-free protease inhibitor tablet (Roche)). The suspension was sonicated for 4 × 60 s and centrifuged for 30 min at 11,000 rpm at 4 °C. The supernatant supplemented with 15 mM imidazole and 1 mM MgCl2 was applied onto a Ni-NTA column (Novagen) pre-equilibrated with lysis buffer. The column was washed with low salt, high salt buffers (30 mM KCl, 50 mM HEPES pH 7.5, 5 mM 2-mercaptoethanol and 300 mM KCl, 50 mM HEPES pH 7.5, 5 mM 2-mercaptoethanol respectively) and low salt buffer containing 50 mM imidazole to remove contaminants, the proteins were eluted with 500 mM imidazole dissolved in low salt buffer. The eluted samples were dialysed against buffer A (300 mM NaCl, 50 mM HEPES pH 7.5, 5 mM MgCl2, 10 mM 2-mercaptoethanol), as a second purification step concentrated and gel-filtrated in buffer A on a Superdex 200 Increase 10/300 GL column (Cytiva).
The GST-tagged Stl protein variants containing cell pellets were resuspended in 30 ml Stl buffer (20 mM TRIS pH 7.5, 300 mM NaCl, 5 mM MgCl2) supplemented with 0.1% Triton X-100, 2 mM dithiothreitol, one cOmplete ULTRA EDTA-free protease inhibitor tablet. The suspension was sonicated for 4 × 60 s and centrifuged for 30 min at 10,000 g at 4 °C. The supernatant was loaded to Glutathione Sepharose 4B resin (GE Healthcare) containing chromatography column pre-equilibrated with Stl buffer. Then the column was washed with 10 bed volumes of Stl buffer. The Stl variant was eluted by overnight cleavage of the GST-tag using 40 units of thrombin in 3 ml Stl buffer at 20 °C.
The Avi-tagged, biotinylated proteins used as sensor proteins for biolayer interferometry measurements were expressed in E. coli AVB101 cells (Avidity, LLC), which contain an inducible BirA gene coding pBirAcm plasmid allowing biotin ligase overexpression in THY media, and induced by 0.5 mM Isopropyl β-D-1-thiogalactopyranoside. The genes encoding Avi-MtDUTWT, Avi-MtDUTΔloop, Stl-Avi and StlNT-Avi proteins were expressed from a pAN4 vector for 4 h at 37 °C and 30 °C. Cells were harvested by centrifugation for 30 min at 4 °C at 4000 rpm, then resuspended in PBS and centrifuged again for 30 min at 4 °C at 4000 rpm. Then cell pellets were frozen and stored at -80 °C. The proteins were purified similarly as previously described, with the exception that the buffers used for the suspension and lysis of the pellets were supplemented with 50 mM biotin, and the cell suspension was incubated for 30 min at 4 °C, in order to allow biotinylation of Avi-tagged proteins.
Protein fractions collected during the purification processes were analysed by SDS-PAGE (Supplementary Figure S15). Protein concentrations were determined based on absorbance values measured at 280 nm by NanoDrop 2000c (Thermo Scientific).
Biolayer interferometry
The kinetics analysis of complex formation between MtDUT enzyme variants (WT and and Δloop mutant) and StlWT and StlNT were carried out by biolayer interferometry using Octet K2 system (FortéBio) at 30 °C. For the equilibration of the high precision streptavidin biosensors (Sartorius), the record of Baseline and the dilution of sensor proteins, Stl buffer (20 mM TRIS pH 7.5, 300 mM NaCl, 5 mM MgCl2) was used. The biotinylated Avi-tagged MtDUT or Stl protein variants were immobilised on the biosensor at 200 µg/ml or 60 µg/ml concentration respectively. The complex formation was investigated from both directions. The experimental setup was designed using Octet Data Acquisition software, the data analysis and the calculation of kinetics parameters (dissociation constant (KD), association (ka) and dissociation (kd) rate constants) were performed using Octet Data Analysis software.
DUT enzyme activity assay
Proton release during the dUTP hydrolysis was followed continuously in activity buffer (1 mM HEPES pH 7.5, 150 mM KCl, 5 mM MgCl2 and 40 µM phenol red) at 559 nm at 20 °C using 10 mm path length cuvette30,31. The pH change during enzymatic reaction is minimal, which does not considerably affect the enzyme activity. The absorbance decrease as a function of time was detected using a Specord 200 (Analylitc Jena) spectrophotometer. The reaction was started with the addition of 10 µM dUTP after 5 min preincubation of 100 nM MtDUT enzyme variants with varying concentrations of StlWT or StlNT in activity buffer at 20 °C. Initial velocity (v0) and turnover number (kcat) were determined from the first 10% of the progress curve12. StlWT and StlNT inhibition data were fitted to the quadratic binding equation13.
Crystallization and data collection, model building & refinement
The crystallization experiments were performed in 54-well sitting drop plates by vapor diffusion method. The initial crystallization conditions were screened in 96-well plates using PACT, JCSG +, Proplex (Molecular Dimensions) commercial reagents. The crystallization droplets were created by mixing the protein solution and the corresponding reservoir solution in a 1:1 volume ratio (0.5:0.5 or 1.0:1.0 µl), the reservoir contained 100 µl crystallization condition. The MtDUT-StlNT complex (protein 6.0 mg/ml) was crystallized with resevoir solution containing 20% PEG 4000, 0.1 M TRIS pH 8.0, wich was supplemented with 25% glycerol as a cryo-protectant before freezing in liquid nitrogen. The MtDUTΔloop enzyme variant (protein solution 13.5 mg/ml) crystals were grown in presence of 5 mM α,β-imido-dUTP (dUPNPP) with reservoir solution of 1.5 M Ammonium sulfate, 0.1 M TRIS pH 8.0, 12% glycerol. The diffraction data sets were collected from single crystals at 100 K on the XRD2 beamline of Elettra Sincrotrone Trieste at wavelength of 1.000 Å. Data were processed using XDS and XSCALE programs39 in case of MtDUTWT-StlNT complex. Resolution cutoff was based on CC1/2 > 0.3 criteria. The structure was solved by molecular replacement using MOLREP40, the structures of M. tuberculosis DUT (PDB ID: 2PY4) and shrimp DUT in complex with Stl (PDB ID: 7DLV) as models. The data from measurement of MtDUTΔloop crystal were processed by iMosflm41 and SCALA from CCP4 suite42. The structure was solved using MOLREP40 and the structure of M. tuberculosis DUT (PDB ID: 2PY4) as a model. The model building and refinement were carried out with Coot43 and Phenix44 (versions 1.19.2_4158-000, 1.20.1_4487) softwares to determine the structures in both cases. The Ramachandran statistics of MtDUTWT-StlNT complex structure are: 96.11% favoured, 3.82% allowed, 0.00% outliers. The Ramachandran statistics of MtDUTΔloop structure are: 99.28% favoured, 0.72% allowed, 0.00% outlier. The data collection and refinement statistics are shown in Supplementary Table S3. The crystal structures have been deposited in Protein Data Bank45 under accession code 8P8O for MtDUTWT-StlNT complex and 8CGA for MtDUTΔloop.
Size exclusion chromatography
An AKTA FPLC purification system with a Superdex 200 Increase 10/300 GL column (GE Healthcare) was used for the size exclusion chromatography measurements. MtDUTWT, MtDUTΔloop, StlNT and StlWT proteins were gel filtrated individually and the 1:1 monomeric molar ratio of MtDUT variants with StlWT or StlNT were injected on the column in Stl buffer. Peak fractions of proteins and protein complexes were collected and analysed by SDS-PAGE, the peak elution volumes were plotted and compared.
Electrospray ionization mass spectrometry
A Waters QTOF Premier mass spectrometer (Waters, Milford, MA, USA) equipped with electrospray ionization source (Waters, Milford, MA, USA) operated in positive ion mode was used to study the MtDUTWT-StlWT protein complex. Proteins were mixed in ca. 20 µM final concentrations and subjected to buffer exchange to 200 mM NH4HCO3 buffer applying VivaspinⓇ 500 Polyethersulfone centrifugal concentrators of 10 kDa weight cutoff. Mass spectra were measured under native conditions: the ions were generated from aqueous 50 mM NH4HCO3 buffer solution (pH 7.5) containing MtDUTWT, StlWT or both protein constructs at ca. 0.5 µM monomer concentration. The capillary voltage was set to 2800 V, the sampling cone voltage was 25 V and the temperature of the source was kept at 80 °C. Collision cell pressure was 3.43 × 10-3 mbar and ion guide gas flow was 35 ml/min. Mass spectra was recorded using the software MassLynx 4.1 (Waters, Milford, MA, USA) in the 1000–8000 m/z mass range.
Mass photometry analysis of complex formation
Microscope cover glasses (No 1.5 H, 24 × 50 mm, Paul Marienfeld GmbH, Lauda-Königshofen, Germany) were cleaned by sequential wash with MQ-water and HPLC-grade isopropanol, 5 times each, followed by drying in a nitrogen stream. Silicon gasket was then fixed on a clean cover glass. Mass calibration was performed for each experiment. Native protein marker (InvitroGen, San Diego, USA) was used to generate mass calibration curve consisting of proteins at MW 66, 146, 480, and 1048 kDa. Immediately prior measurements, protein stocks were diluted directly in Stl buffer (20 mM TRIS pH 7.5, 300 mM NaCl, 5 mM MgCl2) to 100 nM concentration. Individual proteins and complexes were generally measured in mass photometry at 5 nM concentration. For each acquisition, a new well in a silicon gasket was used and 15-μL of sample was introduced into a well. Following autofocus stabilization, movie was recorded for 90s at room temperature. All data acquisition was performed using AcquireMP software (Refeyn Ltd, Oxford, UK) and data was analyzed using DiscoverMP (Refeyn Ltd, Oxford, UK). Data was presented as kernel density estimates with a 5 kDa bandwidth.
Native polyacrylamide gel electrophoresis
The native polyacrylamide gel electrophoresis measurements were carried out using 8% polyacrylamide gels pre-equilibrated with ELFO buffer (25 mM TRIS pH 7.9, 77 mM glycine) for 1.5 h at 150 V. The StlWT or StlNT protein samples were mixed with MtDUTWT protein in 3:3, 2:3 and 1:3 mixing ratios based on monomer concentrations (4.5 µM: 4.5 µM, 3.6 µM:5.4 µM and 2.25 µM:6.75 µM for StlWT:MtDUTWT mixtures and 9 µM:9 µM, 6 µM:9 µM and 3 µM:9 µM for StlNT: MtDUTWT mixtures, respectively) and incubated on ice for 30 min. Then 15 µl of samples were loaded into each well and the electrophoresis was performed for 3 h at 150 V at 4 °C on ice.
Protein structure prediction
To further investigate the mobility of C-terminal arm of MtDUTWT and MtDUTΔloop 10–10 models were generated using AlphaFold246,47. The full-length MtDUT sequence (UniProt: P9WNS5) for MtDUTWT and the same sequence lacking A133-S137 sequence element for MtDUTΔloop were used as an input with 3 copies. 5 relaxed models were generated in each run with template mode set to none. Structural models were superimposed for each enzyme variants.
To analyse the possible role of the C-terminal region of StlWT in interaction with MtDUT we used AlphaFold348 (https://alphafoldserver.com) as a prediction software. 5–5 models were generated using the full-length StlWT (UniProt: Q9F0J8), MtDUTWT (UniProt: P9WNS5) and MtDUTΔloop (UniProt: P9WNS5 lacking A133-S137 sequence element) sequences as input with 3–3 copies.
Protein–protein interaction analysis
The analysis of intermolecular interactions in MtDUTWT-StlNT crystal structure (PDB ID: 8P8O) was performed using PDBePISA (https://www.ebi.ac.uk/pdbe/pisa/pistart.html)49, and LigPlot + v.2.2.850 softwares.
Softwares used to create figures
BLI graphs were created using Octet Data Analysis software (FortéBio). The other graphs were created using Origin 2018 software. Gel images were captured using Image Lab 4.1 software (Bio-Rad). The illustrations of crystal structures were created using PyMOL 2.5.4 (Schrodinger, LLC), the structural superposition of crystal structures was carried out using align command in PyMOL, and electrostatic potential molecular surfaces were created using APBS Electrostatics Plugin51. Illustrations and creation of figures from individual graphs were prepared using CorelDRAW 2020 (Corel Corporation).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful for Edit Hirsch and for the Pharmatech Model Laboratory of Budapest University of Technology and Economics for providing opportunity to perform our BLI experiments. We acknowledge Elettra Sincrotone Trieste for providing access to its synchrotron radiation facilities and we thank Annie Heroux for assistance in using beamline XRD2. The authors thank Péter Tompa for the generous gift of pAN4 vector and E.coli AVB101 cells.
Author contributions
Study design: Z.S.T., A.B., K.N., B.G.V.Performed experiments: Z.S.T., A.B., K.N., I.L., O.O., T.J., M.B.A., R.L.M.Interpreted data: Z.S.T., A.B., K.N., I.L., G.N.N., V.H., B.G.V., T.J., R.L.M., O.O.Wrote article: Z.S.T., A.B., B.G.V., G.N.N.All authors reviewed the manuscript.
Funding
This work was supported by the National Research, Development and Innovation Office of Hungary (K135231, K146890, FK137867, 2018 − 1.2.1-NKP-2018-00005, 2022 − 1.2.2-TÉT-IPARI-UZ-2022-00003 to B.G.V., PD134324 to K.N.) and the TKP2021-EGA-02 grant, implemented with the support provided by the Ministry for Innovation and Technology of Hungary from the National Research, Development and Innovation Office. K.N. was also supported by the Parents Back to Science program of the Budapest University of Technology and Economics. G.N.N. was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences. The X-ray crystallographic study was also supported within projects No. VEKOP-2.3.2-16-2017-00014 and VEKOP-2.3.3-15-2017-00018 (to V.H), of the European Union and the Government of Hungary, co-financed by the European Regional Development Fund; as well as project no. 2018 − 1.2.1-NKP-2018-00005 of the National Research Development and Innovation Fund of Hungary, financed under the 2018 − 1.2.1-NKP funding scheme. This work was also supported by the European Molecular Biology Organization (EMBO) postdoctoral fellowship ALTF 336–2021 (to T.J.). The Novo Nordisk Foundation grant NNF22OC0073736 (to R.L.M.) and the Carlsberg Foundation CF20-0412 (to R.L.M.).
Data availability
The protein crystal structures have been deposited in Protein Data Bank under accession code 8P8O for MtDUTWT-StlNT complex and 8CGA for MtDUTΔloop. Data supporting the findings of this study are available within the paper and its Supplementary Information.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zoé S. Tóth, Email: toth.zoe@ttk.hu
Beáta G. Vértessy, Email: vertessy.beata@ttk.hu
András Benedek, Email: benedek.andras@ttk.hu.
References
- 1.Global tuberculosis report 2023. Licence: CC BY-NC-SA 3.0 IGO (World Health Organization, 2023).
- 2.Singh, V. & Chibale, K. Strategies to combat multi-drug resistance in tuberculosis. Acc. Chem. Res. 54, 2361–2376 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vértessy, B. G. & Tóth, J. Keeping uracil out of DNA: physiological role, structure and catalytic mechanism of dUTPases. Acc. Chem. Res. 42, 97–106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goulian, M. et al. Mechanism of thymineless death. Adv. Exp. Med. Biol., 195(Pt B), 89–95 (1986). [DOI] [PubMed]
- 5.Krokan, H. E., Drabløs, F. & Slupphaug, G. Uracil in DNA–occurrence, consequences and repair. Oncogene. 21, 8935–8948 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Hirmondo, R., Lopata, A., Suranyi, E. V., Vertessy, B. G. & Toth, J. Differential control of dNTP biosynthesis and genome integrity maintenance by the dUTPase superfamily enzymes. Sci. Rep. 7, 6043 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.el-Hajj, H. H., Zhang, H. & Weiss, B. Lethality of a dut (deoxyuridine triphosphatase) mutation in Escherichia coli. J. Bacteriol. 170, 1069–1075 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gadsden, M. H., McIntosh, E. M., Game, J. C., Wilson, P. J. & Haynes, R. H. dUTP pyrophosphatase is an essential enzyme in Saccharomyces cerevisiae. EMBO J. 12, 4425–4431 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pecsi, I. et al. The dUTPase enzyme is essential in Mycobacterium smegmatis. PLoS ONE. 7, e37461 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horváti, K. et al. Antimycobacterial activity of peptide conjugate of pyridopyrimidine derivative against Mycobacterium tuberculosis in a series of in vitro and in vivo models. Tuberculosis (Edinb). 95(Suppl 1), S207–S211 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Nyíri, K. & Vértessy, B. G. Perturbation of genome integrity to fight pathogenic microorganisms. Biochim. Biophys. Acta Gen. Subj. 1861, 3593–3612 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Szabó, J. E. et al. Highly potent dUTPase inhibition by a bacterial repressor protein reveals a novel mechanism for gene expression control. Nucleic Acids Res. 42, 11912–11920 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirmondó, R. et al. Cross-species inhibition of dUTPase via the staphylococcal stl protein perturbs dNTP pool and colony formation in Mycobacterium. DNA Repair. (Amst). 30, 21–27 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Nyíri, K. et al. Structural model of human dUTPase in complex with a novel proteinaceous inhibitor. Sci. Rep. 8, 4326 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benedek, A., Pölöskei, I., Ozohanics, O., Vékey, K. & Vértessy, B. G. The stl repressor from Staphylococcus aureus is an efficient inhibitor of the eukaryotic fruitfly dUTPase. FEBS Open. Bio. 8, 158–167 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benedek, A. et al. The role of a key amino acid position in species-specific proteinaceous dUTPase inhibition. Biomolecules. 9, (2019). [DOI] [PMC free article] [PubMed]
- 17.Tormo-Más, M. A. et al. Moonlighting bacteriophage proteins derepress staphylococcal pathogenicity islands. Nature. 465, 779–782 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dearborn, A. D. & Dokland, T. Mobilization of pathogenicity islands by Staphylococcus aureus strain Newman bacteriophages. Bacteriophage. 2, 70–78 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maiques, E. et al. Another look at the mechanism involving trimeric dUTPases in Staphylococcus aureus pathogenicity island induction involves novel players in the party. Nucleic Acids Res. 44, 5457–5469 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang, F. et al. Structural basis of staphylococcal stl inhibition on a eukaryotic dUTPase. Int. J. Biol. Macromol. 184, 821–830 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Sanz-Frasquet, C., Ciges-Tomas, J. R., Alite, C., Penadés, J. R. & Marina, A. The bacteriophage-phage-inducible chromosomal island arms race designs an interkingdom inhibitor of dUTPases. Microbiol. Spectr. 11, e0323222 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciges-Tomas, J. R. et al. The structure of a polygamous repressor reveals how phage-inducible chromosomal islands spread in nature. Nat. Commun. 10, 3676 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takács, E., Grolmusz, V. K. & Vértessy, B. G. A tradeoff between protein stability and conformational mobility in homotrimeric dUTPases. FEBS Lett. 566, 48–54 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Fiser, A. & Vértessy, B. G. Altered subunit communication in subfamilies of trimeric dUTPases. Biochem. Biophys. Res. Commun. 279, 534–542 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Zang, K., Li, F. & Ma, Q. The dUTPase of white spot syndrome virus assembles its active sites in a noncanonical manner. J. Biol. Chem. 293, 1088–1099 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varga, B. et al. Active site of mycobacterial dUTPase: structural characteristics and a built-in sensor. Biochem. Biophys. Res. Commun. 373, 8–13 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Varga, B. et al. Active site closure facilitates juxtaposition of reactant atoms for initiation of catalysis by human dUTPase. FEBS Lett. 581, 4783–4788 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Nyíri, K., Gál, E., Laczkovich, M. & Vértessy, B. G. Antirepressor specificity is shaped by highly efficient dimerization of the staphylococcal pathogenicity island regulating repressors: stl repressor dimerization perturbed by dUTPases. Sci. Rep. 14, 1953 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan, S. et al. Crystal structure of the Mycobacterium tuberculosis dUTPase: insights into the catalytic mechanism. J. Mol. Biol. 341, 503–517 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Vertessy, B. G. Flexible glycine rich motif of Escherichia coli deoxyuridine triphosphate nucleotidohydrolase is important for functional but not for structural integrity of the enzyme. Proteins. 28, 568–579 (1997). [PubMed] [Google Scholar]
- 31.Vertessy, B. G. et al. The complete triphosphate moiety of non-hydrolyzable substrate analogues is required for a conformational shift of the flexible C-terminus in E. coli dUTP pyrophosphatase. FEBS Lett. 421, 83–88 (1998). [DOI] [PubMed] [Google Scholar]
- 32.Nagy, G. N., Leveles, I. & Vértessy, B. G. Preventive DNA repair by sanitizing the cellular (deoxy)nucleoside triphosphate pool. FEBS J. 281, 4207–4223 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Nagy, G. N. et al. Structural characterization of Arginine fingers: identification of an Arginine Finger for the pyrophosphatase dUTPases. J. Am. Chem. Soc. 138, 15035–15045 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Nyíri, K. et al. HDX and native mass spectrometry reveals the different structural basis for interaction of the Staphylococcal pathogenicity island repressor Stl with dimeric and trimeric phage dUTPases. Biomolecules. 9, (2019). [DOI] [PMC free article] [PubMed]
- 35.Lopata, A. et al. A hidden active site in the potential drug Target Mycobacterium tuberculosis dUTPase is accessible through small amplitude protein conformational changes. J. Biol. Chem. 291, 26320–26331 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tóth, J., Varga, B., Kovács, M., Málnási-Csizmadia, A. & Vértessy, B. G. Kinetic mechanism of human dUTPase, an essential nucleotide pyrophosphatase enzyme. J. Biol. Chem. 282, 33572–33582 (2007). [DOI] [PubMed] [Google Scholar]
- 37.García-Nafría, J., Timm, J., Harrison, C., Turkenburg, J. P. & Wilson, K. S. Tying down the arm in Bacillus dUTPase: structure and mechanism. Acta Crystallogr. D Biol. Crystallogr. 69, 1367–1380 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Nord, J., Nyman, P., Larsson, G. & Drakenberg, T. The C-terminus of dUTPase: observation on flexibility using NMR. FEBS Lett. 492, 228–232 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Kabsch, W. X. D. S. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vagin, A. & Teplyakov, A. Molecular replacement with MOLREP. Acta Crystallogr. D Biol. Crystallogr. 66, 22–25 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Battye, T. G. G., Kontogiannis, L., Johnson, O., Powell, H. R. & Leslie, A. G. W. iMOSFLM: a new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr. D Biol. Crystallogr. 67, 271–281 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agirre, J. et al. The CCP4 suite: integrative software for macromolecular crystallography. Acta Crystallogr. D Struct. Biol. 79, 449–461 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D Struct. Biol. 75, 861–877 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berman, H. M. et al. The protein data bank. Nucleic Acids Res. 28, 235–242 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature. 596, 583–589 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mirdita, M. et al. ColabFold: making protein folding accessible to all. Nat. Methods. 19, 679–682 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abramson, J. et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature. 630, 493–500 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krissinel, E. & Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Laskowski, R. A. & Swindells, M. B. LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 51, 2778–2786 (2011). [DOI] [PubMed] [Google Scholar]
- 51.Jurrus, E. et al. Improvements to the APBS biomolecular solvation software suite. Protein Sci. 27, 112–128 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The protein crystal structures have been deposited in Protein Data Bank under accession code 8P8O for MtDUTWT-StlNT complex and 8CGA for MtDUTΔloop. Data supporting the findings of this study are available within the paper and its Supplementary Information.