Abstract
Experiencing a hurricane during pregnancy is associated with increased risk of adverse birth outcomes and poor mental health. Pregnant people from marginalized communities are more susceptible to adverse effects, as they have fewer resources to overcome hardships at a time when shelter and nutrition are essential. When Hurricane Maria (HM) devastated Puerto Rico in September 2017, the archipelago was already burdened with high poverty, health disparities, environmental contamination, and fragile utility infrastructure. We aimed to compare biomarkers of environmental exposures among pregnant participants in the PROTECT cohort before and after HM and to identify hurricane-related sources of exposure. Metals, PAHs, phthalate metabolites, and phenols were measured in urine samples collected from participants at three prenatal visits (2011–2019). Samples were categorized as before, <3 months, 3–6 months, and >6 months after HM. Using linear mixed effects models, we found that in the 6 months after HM, participants had higher Co, Ni, and DEHPTP concentrations, indicating increased exposure, and lower concentrations of PAHs, several metals, and phthalates, suggesting decreased exposure, compared to pre-HM levels. Biomarkers were not associated with potential exposure sources assessed through questionnaire or previously measured tap water contaminants. This study provides insight into how extreme weather events may alter environmental exposures among pregnant people in Puerto Rico. As climate change has increased the frequency and magnitude of such events, additional research is needed to clarify the implications for maternal and child health and to identify sources of related environmental exposures within this vulnerable population.
Keywords: Puerto Rico, Hurricane Maria, Extreme weather, Climate change, Environmental exposures, Pregnancy
Highlights
-
•
We compared prenatal exposures in Puerto Rico before and after Hurricane Maria.
-
•
Exposure to Co, Ni, Tl, and phthalate replacements was higher after Hurricane Maria.
-
•
Exposure to PAHs, several metals, and phthalates was lower after Hurricane Maria.
-
•
Exposure was not associated with experiences during Maria or tap water contaminants.
-
•
Extreme weather events may alter environmental exposures among pregnant people.
1. Introduction
Climate change has increased the frequency and severity of hurricanes and other extreme weather events [1,2] with critical implications for human health. Beyond direct injuries and loss of life, experiencing a hurricane is associated with increased risk of infectious disease, cardiac and respiratory events, and poor mental health [3]. People who are pregnant are especially vulnerable, as experiencing a hurricane during pregnancy is associated with higher risk for adverse birth outcomes such as stillbirth, preterm birth, and low birth weight babies, as well as anxiety, depression, and post-traumatic stress disorder [[4], [5], [6], [7]]. Experiencing a hurricane may limit one's ability to access food, clean water for drinking, cooking, and washing, and may increase exposure to environmental hazards, such as chemicals or mold, all of which have critical implications for birth outcomes [[5], [6], [7]].
Pregnant people from marginalized communities are even more susceptible to the adverse effects of hurricanes, as they have fewer resources to overcome hardships at a time when shelter and nutrition are essential [8,9]. For example, Puerto Rico is highly vulnerable to hurricanes because of its location in the Caribbean, high poverty rate (estimated at 40 % in 2022) [10], substantial health disparities [[11], [12], [13]], widespread environmental contamination [14], and fragile utility and healthcare infrastructure [15]. Indeed, Puerto Rico was devastated when Hurricanes Irma and Maria hit in September 2017. We previously evaluated the impact of Hurricane Maria on participants in the PROTECT study, an ongoing birth cohort in northern Puerto Rico. In Maria's aftermath, the median number of days without electricity for pregnant participants was 90 days, 80 % lost cell phone service, and many reported structural or flood damage to their home [16], and challenges accessing prenatal care [17].
Environmental exposures are an important concern, as hurricanes bring flooding, landslides, and structural damage that can exacerbate or spread existing environmental contamination [18,19]. Puerto Rico is particularly vulnerable to hurricane-related contamination, as there are over 200 registered hazardous waste sites, including 19 active Superfund sites, across the archipelago [20]. Environmental contamination from these sites can be distributed through karst aquifers, potentially resulting in increased exposure risk during extreme weather events [14,21]. In Puerto Rico, a study of drinking water quality after Hurricane Maria reported elevated concentrations of 34 trace elements and environmental pollutants, including arsenic, nickel, manganese, copper, per- and polyfluoroalkyl substances, and the herbicide atrazine, in tap water samples, compared to samples collected before Maria, although levels did not exceed health-based benchmark concentrations [21,22]. Changes in behavior after a hurricane may also lead to changes in environmental exposures. For example, we previously reported that in the aftermath of Hurricane Maria, many PROTECT participants had to seek alternative sources for food and clean water and many used diesel-powered generators [16], which could potentially lead to increased exposures.
These findings highlight the need to characterize hurricane-related exposures to environmental contaminants, particularly among vulnerable populations. We have utilized biological samples collected from participants in the PROTECT birth cohort to assess changes in exposure biomarker concentrations from before Hurricane Maria, during the months-long recovery period, and up to two years after the event.
2. Materials and methods
2.1. Study population
Participants were pregnant women enrolled in the PROTECT project between 2011 and 2019. PROTECT is a prospective birth cohort in Puerto Rico testing the hypothesis that exposure to environmental contamination contributes to higher risk of preterm birth. Inclusion criteria for PROTECT include residing in the Northern karst region, age between 18 and 40 years, no gestational or medical complications (e.g., preexisting diabetes, hypertension, liver/kidney disease, multiple gestations), no use of oral contraceptives three months before pregnancy, and no pregnancies stemming from assisted reproductive technologies. Women are recruited into the PROTECT study at their first prenatal visit to participating clinics at less than 16 weeks gestation (median 14 weeks). Study protocols were approved by the research and ethics committees of the University of Puerto Rico Medical Sciences Campus, Northeastern University, and the University of Michigan School of Public Health. All participants provided informed consent prior to participation.
2.2. Study design
Participants attended prenatal study visits at 16–20 (Visit 1), 20–24 (Visit 2), and 24–28 (Visit 3) weeks gestation. At each visit, participants provided spot urine samples and information on medical history, sociodemographic characteristics, diet, and product use through extensive interview questionnaires. Detailed medical information was also collected via systematic abstraction of medical record data. A range of urinary biomarkers of environmental exposures are measured in samples collected from PROTECT participants at each study visit, including phthalate metabolites, phenols, metals, and PAHs.
In the PROTECT “Hurricane Study”, we utilized existing PROTECT data and infrastructure to assess hurricane-related changes in exposure to environmental contaminants by comparing urinary concentrations in samples collected before and after Hurricanes Irma and Maria. In addition, PROTECT participants who were pregnant during Hurricane Maria or became pregnant in the 5 months after Hurricane Maria were also contacted between August 2018 to March 2019 to participate in a phone-administered questionnaire to collect information on their experiences during Hurricane Maria.
2.3. Urinary biomarkers of environmental exposures
Urine samples were collected into polypropylene containers, labeled, and placed on ice for transport to the University of Puerto Rico (UPR) Medical Sciences Campus laboratory. Urinary specific gravity was then measured using a hand-held digital refractometer (Atago Co., Ltd., Tokyo, Japan) to account for urine dilution. Samples were divided into 2 mL aliquots and frozen at −80C until overnight shipment on dry ice to laboratories for analysis or the University of Michigan for storage (−80C). All samples included in this analysis were collected between 2011 and 2019. For all analytes, concentrations below the limits of detection (LOD) were imputed with the LOD divided by the square root of 2 [23].
2.4. PAHs
Urinary concentrations of hydroxylated PAH metabolites (OH-PAHs) were measured at NSF International (Ann Arbor, MI, USA) using liquid chromatography-mass spectrometry coupled with online solid phase extraction as previously described [24,25]. Measured OH-PAHs were 1-hydroxynapthalene (1-OH-NAP), 2-hydroxynapthalene (2-OH-NAP), 2-hydroxyfluorene (2-OH-FLU), 1-hydroxyphenanthrene (1-OH-PHE), the sum of 2-hydroxyphenanthrene and 3- hydroxyphenanthrene (Σ2,3-OH-PHE), 4-hydroxyphenanthrene (4-OH-PHE), 9-hydroxyphenanthrene (9-OH-PHE), and 1-hydroxypyrene (1-OH-PYR).
2.5. Metals
Urinary metals and metalloids were measured at NSF International (Ann Arbor, MI, USA) using inductively-coupled plasma mass spectrometry (ICPMS) as described previously [26]. Concentrations of 21 metals and metalloids were measured, comprising Arsenic (As), barium (Ba), beryllium (Be), cadmium (Cd), cobalt (Co), chromium (Cr), cesium (Cs), copper (Cu), mercury (Hg), manganese (Mn), molybdenum (Mo), nickel (Ni), lead (Pb), platinum (Pt), antimony (Sb), tin (Sn), titanium (Ti), tungsten (W), uranium (U), vanadium (V), and zinc (Zn).
2.6. Phenols
Urinary concentrations of 2,4-dichlorophenol (2,4-DCP), 2,5-dichlorophenol (2,5-DCP), bisphenol A (BPA), bisphenol S (BPS), bisphenol F (BPF), benzophenone-3 (BP-3), triclosan (TCS), triclocarban (TCC), ethylparaben (E-PB), methylparaben (M − PB), butylparaben (B-PB), and propylparaben (P-PB) were measured at the Centers for Disease Control and Prevention (CDC) laboratory (Atlanta, GA, USA) using online solid phase extraction-high-performance liquid chromatography-isotope dilution tandem mass spectrometry [27,28].
2.7. Phthalates
Urinary concentrations of phthalate and phthalate replacement metabolites were measured at the CDC laboratory using online solid phase extraction high-performance liquid chromatography–isotope dilution tandem mass spectrometry, as described in detail elsewhere [29]. Samples were analyzed for up to 16 phthalate metabolites. Mono-2-ethylhexyl phthalate (MEHP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), mono-2-ethyl-5-carboxypentyl phthalate (MECPP), monoethyl phthalate (MEP), mono-n-butyl phthalate (MBP), monobenzyl phthalate (MBzP), mono-isobutyl phthalate (MiBP), mono-3-carboxypropyl phthalate (MCPP), mono carboxyisononyl phthalate (MCNP), and mono carboxyisooctyl phthalate (MCOP) were included in all batches. Phthalate metabolites mono-hydroxyisobutyl phthalate (MHiBP) and mono-hydroxybutyl phthalate (MHBP), and then mono-oxononyl (MONP) and terephthalate metabolites mono-2-ethyl-5-carboxypentyl terephthalate (MECPTP) and mono-2-ethyl-5-hydrohexyl terephthalate (MEHHTP) were added to the analytical panel in later batches.
2.8. Protect questionnaires
PROTECT questionnaires given to all participants at each study visit combine elements from the National Health and Nutrition Examination Survey (NHANES), the National Health Interview Survey, the Birth Defects Prevention Study, and the National Children's Study, with modifications to capture important local Puerto Rico patterns or variables. These questionnaires were translated into Spanish by bilingual native Puerto Rican Spanish speakers, and were used to collect information on demographics, maternal education, income, BMI, smoking history, and reproductive history. At each study visit, participants also answered questions regarding recent food consumption, drinking water sources, and product use.
2.9. Hurricane experience questionnaire
PROTECT participants who were pregnant during Hurricane Maria or became pregnant and enrolled in PROTECT within 5 months after Maria were eligible for this sub-study. To characterize experiences that PROTECT women may have had either during Hurricane Maria or during the recovery period, we designed and administered a hurricane experience questionnaire in Puerto Rican Spanish via a phone call. Participants were asked to answer questions on storm-related damage to housing, injuries to participants, families, and friends, illness, power availability, availability and sources of food and water, pesticide exposure, noise, financial difficulties, and medical and prenatal care based on their experiences in the immediate aftermath of Hurricane Maria.
2.10. Statistical analysis
Prior to data analyses, urinary biomarker concentrations were corrected for specific gravity to adjust for urinary dilution using the equation Pc = P (1.019-1)/(SG-1)
where Pc = corrected metabolite concentration, P = measured metabolite concentration, SG = specific gravity of the sample, and 1.019 = median specific gravity of all samples collected. Corrected analyte concentrations were right skewed, and therefore natural log-transformed to obtain a normal distribution. For analytes with concentrations below the LOD in greater than 50 % of samples, a dichotomous, analyte specific detect vs. non-detect variable was created.
A categorical variable (“Hurricane Status”) was created to designate the time between Hurricane Maria and each PROTECT study visit: 1) visit occurred before Hurricane Maria (pre-Maria); 2) visit occurred within 3 months after Hurricane Maria (<3 months); 3) visit occurred between 3 and 6 months after Hurricane Maria (3–6 months); and 4) visit occurred 6 or more months after Hurricane Maria (>6 months). There were only 4 urine samples with measured phenols and parabens in the 3–6 months’ time period, so the <3 and 3–6 month categories were collapsed for analyses with these chemicals.
Geometric means and select percentiles were calculated for each analyte, stratified by Hurricane Status time period. Hurricane Status variables were also entered into linear mixed effect models as categorical predictors of urinary exposure biomarker concentrations while controlling for within individual correlations over time (i.e. a random intercept was included in all models for individual ID). Effect estimates are presented as the percent change in geometric means of a specific biomarker from pre-Maria to either <3, 3–6, or >6 months post-Maria. For chemicals that were categorized only as detect vs. non-detect, we used generalized linear mixed models with a logit link function to determine Hurricane Status as a predictor of biomarker detection. Maternal education and income were included as covariates in all models. To assess specific sources of exposures in the 6 months after Hurricane Maria, we used linear mixed effect models to investigate associations between responses on the Hurricane Experience Questionnaire and urinary biomarker concentrations among PROTECT participants during this same time period. Regression diagnostics including residual plots were assessed for all analyses to evaluate model fit, and all analyses were performed using SAS 9.4.
3. Results
In the 6 months after Hurricane Maria, 95 participants provided a urine sample at 177 separate study visits for analysis of exposure biomarkers. Urinary phthalate metabolites were measured in the highest number of samples, with measurements for 67 urine samples collected within 3 months after Maria, 110 samples collected between 3 and 6 months after Maria, and 941 samples collected more than 6 months after Maria (Table S1). PAHs and metals were measured in the majority of these samples (Tables S2 and S3 respectively), while at the time of data analysis, phenols and parabens were measured in only a small number of samples collected in the six months after HM (<3 months n = 21, 3–6 months n = 4; Table S4). The analytes 4-OH-PHE, 9-OH-PHE, Cr, Be, Tl, U, W, B-PB, E-PB, BPF, MHINCH, MCOCH, and MNP were detected in less than 50 % of analyzed samples and thus were examined as detect vs. non-detect (Table 1).
Table 1.
Differences in the percent of samples with detectable analyte concentrations within 3, 3–6, and >6 months after Hurricane Maria compared to pre-hurricane levels.
| PRE-MARIA |
<3 MONTHS |
3 to <6 MONTHS |
>6 MONTHS |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % > LOD | N | % > LOD | p-valuea | N | % > LOD | p-valuea | N | % > LOD | p-valuea | |
| PAHs | |||||||||||
| 4-OH-PHE | 1750 | 66.7 | 66 | 40.9 | 0.004 | 88 | 31.8 | <0.0001 | 460 | 70.2 | 0.75 |
| 9-OH-PHE | 1750 | 46.6 | 66 | 31.8 | 0.07 | 88 | 22.7 | 0.001 | 460 | 47.4 | 0.86 |
| Metals | |||||||||||
| Cr | 1902 | 19.2 | 66 | 21.2 | 0.62 | 97 | 2.1 | 0.005 | 466 | 7.5 | <0.0001 |
| Be | 1902 | 23.1 | 66 | 7.6 | 0.02 | 97 | 15.5 | 0.41 | 466 | 7.3 | <0.0001 |
| Tl | 1902 | 46.8 | 66 | 69.7 | 0.008 | 97 | 67 | 0.001 | 466 | 49.6 | 0.13 |
| U | 1902 | 36.2 | 66 | 9.1 | 0.0002 | 97 | 9.3 | <0.0001 | 466 | 9.7 | <0.0001 |
| W | 1902 | 22.9 | 66 | 18.2 | 0.73 | 97 | 4.1 | 0.002 | 466 | 2.4 | <0.0001 |
| Phenolsb | |||||||||||
| B-PB | 2584 | 53.2 | 25 | 60.0 | 0.84 | 640 | 37.2 | <0.0001 | |||
| E-PB | 1945 | 53.4 | 25 | 72.0 | 0.26 | 640 | 36.9 | <0.0001 | |||
| BPF | 1752 | 44.9 | 25 | 32.0 | 0.14 | 640 | 47.4 | 0.17 | |||
| Phthalates | |||||||||||
| MHINCH | 2620 | 36.5 | 66 | 47.8 | 0.14 | 110 | 30.9 | 0.18 | 941 | 54.1 | <0.0001 |
| MCOCH | 2620 | 21.1 | 66 | 32.8 | 0.05 | 110 | 12.7 | 0.20 | 941 | 27.7 | 0.002 |
| MNP | 2620 | 29.6 | 66 | 26.9 | 0.71 | 110 | 27.3 | 0.11 | 941 | 27.3 | 0.22 |
LOD, limit of detection; N = number of urine samples collected at study visits during the specified time frame.
P-values from mixed models accounting for within person correlation across samples and adjusted for income and education, with samples collected before Hurricane Maria as the reference group.
Phenols were measured in only 4 samples collected 3 to <6 months post-Maria, so the <3 month and 3 to <6 months categories were combined (n = 25).
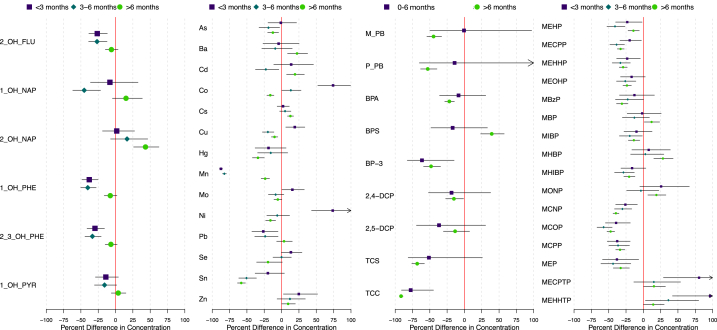
The percent change in urinary concentrations of each analyte from before Maria to <3 months, 3–6 months, and >6 months after Maria are presented in Fig. 1. Geometric means (GM) and select percentiles of urinary PAH concentrations during each Hurricane Status time period are presented in Table S2. The GMs of several urinary PAH concentrations were significantly lower in the <3 and 3–6 months after Maria (Fig. 1), including 2-OH-FLU (% Difference <3 months = −26 % (95%CI = −38,-12); 3–6 months = −27 % (−39,-12)); 1-OH-NAP (3–6 = −45 % (−62, −22)), 1-OH-PHE (<3 = −38 % (−48, −25); 3–6 = −40 % (−50, −28)), and 2,3-OH-PHE (<3 = −30 % (−41, −16); 3–6 = −33 % (−44, −21)). After 6 months, urinary PAH concentrations returned to pre-Maria levels, or in the case of 2-OH-NAP, were significantly higher (>6 = 43 % (26, 63)).
Fig. 1.
Percent difference in geometric mean urinary concentrations of A) PAHs, B) metals, C) phenols and parabens, and D) phthalate metabolites, in samples collected from PROTECT participants within 3, 3–6, and >6 months after Hurricane Maria compared to pre-hurricane levels (% change, 95 % CI).
The change in urinary metal concentrations from before to after Maria varied considerably depending on the metal and how long after Maria the sample was collected (Fig. 1; Table S3). For example, Co and Ni both were dramatically higher (74 % (95%CI: 52, 99) and 74 % (95%CI: 43, 111), respectively) in the <3 months after Maria, returned to almost pre-Maria concentrations in the 3–6 months period, then were significantly lower in the >6 months after Maria. In contrast, urinary Mn was dramatically lower in the <3 months and 3–6 months after Maria (−87 % (95%CI: 90, −85); −83 % (95%CI: 85, −80), respectively, and although still significantly lower, concentrations began to move towards pre-Maria levels after >6 months (−24 % (95%CI: 30, −17)). Cu and Zn were also significantly higher after Maria, but only in the first 3 months. Urinary As, Pb, and Sn were lower in at least one time period after Maria.
Lower urinary concentrations of BP-3 and TCC were the only significant changes in phenols or parabens in the 0–6 months after Maria (Fig. 1, Table S4). With the exception of BPS, all phenols and parabens were lower in the period >6 months after Maria, although 2,5-DCP was not statistically significant. In contrast, BPS was significantly higher in the >6 month after Maria period (39 %, 95%CI: 23, 57).
In the 3 months after Maria, urinary DEHP metabolites, MCNP, MCOP, MCPP, and MEP were all significantly lower, while metabolites of DEHTP were significantly higher, compared to pre-Maria levels (Fig. 1. Table S1). This trend continued in the 3–6 month and >6 month period after Maria, with several phthalate metabolite concentrations continuing to be lower after Maria. Exceptions to this trend included MBP and MONP, which didn't significantly change in the <3 and 3–6 month after Maria periods, but were significantly higher in the >6 month period. In addition, DEHPTP metabolites continued to be higher compared to pre-Maria levels in all time periods after Maria.
Regarding analytes that were evaluated as detected vs. not detected, the percentage of urine samples from each Hurricane Status time period with detectable concentrations are presented in Table 1. Both PAHs (4-OH-PHE, 9-OH-PHE) were detected in a lower percentage of samples in the <3 and 3–6 months after Maria time periods, but returned to pre-Maria detection rates in the >6 month period. Tl was the only metal with significantly higher detection rates after Maria, with the percent of samples above the LOD increasing from 46.8 % before Maria to 69.7 % in the <3 months (p = 0.008) and 67.0 % in the 3–6 months (p = 0.001) after Maria. Detection of Tl returned to rates observed before Maria after 6 months (49.6 %, p = 0.13). Detection rates were lower in at least one post-Maria time period for Cr, Be, U, and W, and remained so even after 6 months. Detection rates of B-PB and E-PB were higher in the <6 months after Maria, although our sample size for these analytes in this time period was small (n = 25) and the change was not statistically significant. In contrast, detection of these parabens was significantly lower in the >6 months after Maria, compared to pre-Maria rates. Detection of DINCH metabolites, MHINCH and MCOCH, varied in the <3 and 3–6 months after Maria but were significantly higher in the >6 months after Maria (54.1 %, p < 0.0001; 27.7 %, p = 0.002; respectively) compared to pre-Maria rates (36.5 %; 21.1 %).
Among the 102 PROTECT participants that completed the Hurricane Experience Questionnaire [16], 68 provided a urine sample at a study visit within 6 months after Hurricane Maria (Table 2). Within this group, 100 % reported bottled water as their primary drinking water source, while no one reported drinking tap water. This prevented us from being able to evaluate drinking water as a potential source of environmental exposure after Maria. Most participants had access to adequate food (86.8 %) and generators for their home (83.8 %), although many reported structural damages (45.6 %) or standing water either in or near their home (32.4 %). Participant responses to the Hurricane Experience Questionnaire were not significantly associated with urinary biomarkers of exposure in the 6 months after Hurricane Maria (Table 3). However, higher Mn levels were marginally associated (p < 0.1) with feeling sick after Maria and having trash and/or debris near the home.
Table 2.
Responses to the Hurricane Experience Questionnaire among participants who had at least one exposure measurement from a urine sample collected at a study visit within the 6 months after Hurricane Maria (n = 68).
| YES |
NO |
|
|---|---|---|
| N (%) | N (%) | |
| Bottled Drinking Water | 68 (100) | 0 (0) |
| Tap Water for Drinking | 0 (0) | 68 (100) |
| Felt Sick | 21 (30.9) | 47 (69.1) |
| Access to Food | 59 (86.8) | 9 (13.2) |
| Home: Structural Damage | 31 (45.6) | 37 (54.4) |
| Home: Flood Damage | 21 (30.9) | 47 (69.1) |
| Home: Standing Water In/Nearby | 22 (32.4) | 46 (67.6) |
| Home: Trash/Debris Nearby | 48 (70.6) | 20 (29.4) |
| Home: Generator | 57 (83.8) | 11 (16.2) |
Table 3.
Percent difference in biomarkers of exposure measured in urine samples with 3 months after Hurricane Maria in relation to responses to the Hurricane Experience Questionnaire.
| 1-OH-PHE |
Co |
Mn |
Ni |
|
|---|---|---|---|---|
| % Diff (95 % CI) | % Diff (95 % CI) | % Diff (95 % CI) | % Diff (95 % CI) | |
| Felt Sick | 2 (−25.6, 39.7) | −12.1 (−30.2, 10.7) | 73.5 (−2.7, 210) | −13.4 (−35.4, 16.1) |
| Access to Food | −1.6 (−33.9, 46.5) | 28.7 (−4.8, 74) | 24.2 (−43.2, 172) | 34.5 (−7.8, 96.1) |
| Home: Structural Damage | 0.5 (−25.7, 35.8) | 5.5 (−15.5, 31.8) | −28.3 (−58.9, 25) | 20 (−9.3, 58.9) |
| Home: Flood Damage | 8.8 (−21.8, 51.4) | 8.5 (−14.6, 37.7) | −1.3 (−46.4, 81.9) | 16.8 (−13.6, 58) |
| Home: Standing Water In/Nearby | −5.7 (−31.7, 30.1) | −0.5 (−21.3, 25.7) | 59.4 (−10.6, 184) | −5.4 (−29.9, 27.5) |
| Home: Trash/Debris Nearby | −9.6 (−34.2, 24.3) | −9.1 (−28.6, 15.8) | 67.2 (−8, 204) | −9.4 (−33.4, 23.2) |
| Home: Generator | 16 (−25.9, 81.7) | −22.9 (−45.2, 8.6) | −32.5 (−71.1, 57.9) | −2 (−37.4, 53.2) |
| MECPTP | MEHHTP | P-PB | M-PB | |
| % Diff (95 % CI) | % Diff (95 % CI) | % Diff (95 % CI) | % Diff (95 % CI) | |
| Felt Sick | −32.1 (−60, 15.3) | −17.3 (−49.3, 35) | −64.1 (−99.3, 1710) | −27.4 (−95.4, 1044) |
| Access to Food | 19.9 (−40.8, 143) | 10.6 (−42.1, 111) | 228 (−95.2, 22355) | 39.3 (−93.4, 2842) |
| Home: Structural Damage | 14.7 (−31, 90.6) | 28.4 (−18.9, 103) | −86.2 (−99.4, 244) | −78 (−97.5, 94.7) |
| Home: Flood Damage | −13.4 (−49.9, 49.7) | −16.5 (−49.3, 37.5) | 102 (−94.4, 7202) | 33.6 (−88.9, 1504) |
| Home: Standing Water In/Nearby | 34.9 (−20.3, 128) | 13.2 (−30.7, 84.9) | −0.4 (−97.9, 4582) | 4.7 (−92.5, 1361) |
| Home: Trash/Debris Nearby | −14.8 (−51.4, 49.1) | −19 (−51.4, 35) | −46.8 (−99, 2713) | −40.4 (−95.9, 760) |
| Home: Generator | −11.9 (−58.2, 85.7) | 21.4 (−39.1, 142) | −32 (−99.1, 4985) | −55.9 (−98, 867) |
% Diff: difference in urinary concentrations between people who answered “Yes” compared to “No”; LCL: lower 95 % confidence limit; UCL: upper 95 % confidence limit. Urinary concentrations were ln-transformed, standardized for specific gravity. Bolded results indicate p < 0.1.
4. Discussion
In the wake of Hurricane Maria, PROTECT participants had higher concentrations of Co, Ni, and DEHPTP metabolites, as well as increased detection of Tl and DINCH metabolites, in their urine, indicating increased exposure to these chemicals. However, contrary to our hypotheses, concentrations of many other analytes were lower, suggesting decreased exposure to PAHs, several metals and phthalates, as well as lower or unchanged phenol and paraben exposure, during this same time period. We also were not able to connect changes in biomarker concentrations to potential sources of exposure assessed through participant questionnaire.
Given reports of extensive drinking water contamination in Puerto Rico after Hurricane Maria [21], we anticipated that PROTECT participants would have higher exposure to many contaminants in the 3–6 months following the storm. Specifically, levels of As, Ni, Mn, Cu were higher in tap water samples collected in Northern and Southern Puerto Rico, including households of PROTECT participants, although all concentrations were below health-based benchmark levels [21,22]. Biomarkers of Cu and Ni did briefly increase in the 3 months after Maria, but they then decreased below the pre-Maria levels in the following months. Manganese, which increased by over ten-fold in tap water, drastically decreased in urine samples collected after Maria compared to pre-Maria levels. These findings are consistent with reports from participants in that they did not drink tap water during the recovery period, either because tap water wasn't available or was clearly undrinkable. Participants sought drinking water from other sources, such as various types of bottled water. Interestingly, prior to Hurricane Maria, PROTECT participants had urinary Mn levels approximately ten times higher than women in NHANES age 18–40 years [30]. The drastic drop in urinary Mn after the cessation of drinking tap water, and the increase in Mn present in the tap water samples after Hurricane Maria [21], both suggest that tap water contamination may be a significant source of Mn exposure within this population.
We also hypothesized that PROTECT participants would have higher exposure to PAHs after Maria due to the ubiquitous use of household generators. Again, we observed the opposite, with lower concentrations of PAH exposure biomarkers measured in samples collected after Maria. Lower PAH exposure may be a result of drastic changes in traffic in the immediate aftermath of Maria, as car exhaust is a major source of PAH exposure [31]. Traffic density likely decreased after Maria due to unpassable roads and scarcity of gasoline. Changes in diet may also be an explanation for decreases in PAH exposure, as fresh food was less available during this time, and grilled meats and other foods are a source of PAHs [31].
Except for DEHTP, biomarkers of exposure to several phthalates decreased in the months after Hurricane Maria and stayed lower than pre-Maria levels beyond the 6-month recovery period. In contrast, DEHTP levels increased immediately after Maria, possibly due to an influx of processed and packaged foods. DEHTP metabolites decreased slightly over the following months, but never returned to pre-Maria levels. This shift in the distribution of phthalate biomarkers could be at least partially related to Hurricane Maria, but it could also be due to overall trends in phthalate and phthalate replacement use in products over time [32].
A key strength of this study is the measurement of biomarkers of exposure in urine samples collected both before and after Hurricane Maria. Previous studies of chemical contamination after a hurricane or similar natural disaster have predominantly relied on post-disaster measurements in soil or sediment. For example, a study of soil contamination in New York City after Hurricane Sandy found high levels of lead, arsenic, PAHs, and PCBs in residential areas near known sites of contamination [33]. In the wake of Hurricanes Katrina and Rita, studies reported increased metal concentrations in soil samples collected in New Orleans, LA including Pb and As levels exceeding human health screening values set by the US Environmental Protection Agency [18,34,35]. In Houston, TX extensive flooding during Hurricane Harvey resulted in redistribution of many contaminants, with high levels of PAHs, metals, and PCBs measured in soil and sediment samples [36,37]. Increased concentrations in metals in soil after a hurricane is consistent with our findings of higher urinary concentrations of some metals after Hurricane Maria, although we also saw lower concentrations of other metals and PAHs. These inconsistencies could be due to geographical differences in contaminants or differences in the media used for measurement, as soil levels represent a potential source of exposure, while biomarkers represent exposure from all sources.
One study utilized silicone wristbands among residents of Houston after Hurricane Harvey to screen for exposure to over 1500 chemicals. This study reported higher levels of 35 different chemicals, including PAHs, flame retardants, and pesticides (metals were not measured) in the post-hurricane samples compared to estimated baseline levels [38,39]. Interestingly, this study reported lower concentrations of phthalates after Hurricane Harvey, which is consistent with biomarker concentrations in our study. In contrast, we did not see higher biomarkers of PAH exposure after Hurricane Maria. Again, this inconsistency between studies could be due to differences in area contamination, in post-hurricane conditions, or recovery challenges. For example, we hypothesize that PAH exposure in Puerto Rico decreased after Hurricane Maria due to drastic decreases in traffic pollution. This may not have been the case after Hurricane Harvey, as Houston is not as geographically isolated as Puerto Rico and likely had access to fuel for vehicles and generators more quickly.
This analysis had several limitations, including the small number of urine samples collected in the first three months after Hurricane Maria. This somewhat limited our ability to fully characterize exposures during this important time period, especially when compared to the hundreds of samples collected before and greater than six months after Maria. However, given the conditions in Puerto Rico at the time, resuming study visits with PROTECT participants and collecting any samples within this time period was an important accomplishment for our Puerto-Rico based study team and for the PROTECT community. Another limitation was the amount of time that passed between Hurricane Maria and administration of the exposure questionnaire, particularly because this 12–18 month time period was likely highly stressful. Experiences during this long period may have affected participant recall of specific sources of exposure or events related to exposure. In addition, although there were over 100 participants who completed the exposure questionnaire, only 68 of these participants also had urine samples collected during the 3–6 months after Hurricane Maria for biomarker exposure analysis. Finally, despite efforts to understand potential sources of chemical exposures related to the storm and recovery we have not been able to evaluate connections between hurricane-related exposures and adverse birth outcomes in this population. This is primarily due to the relatively small number of PROTECT participants who gave birth in the 3–6 months after Hurricane Maria, as well as potentially increased rates of loss to follow up during this time [16]. Given these limitations, our findings may not be generalizable to populations beyond women of child-bearing age living in Puerto Rico.
Previous research has shown that people who experience a hurricane during pregnancy are at higher risk for adverse birth outcomes and impacts on mental health [[4], [5], [6], [7]]. However, this work highlights the need for additional research and public health surveillance to identify individuals and communities with increased vulnerability to these health outcomes in the event of a storm, as well as specific causes, such as limited access to clean water, food, and prenatal care, or increased environmental exposures and stress. Future work must inform preparedness efforts for pregnant people, prenatal care practitioners, and disaster response in Puerto Rico, and is more critically important now than ever. Since Hurricane Maria struck Puerto Rico in 2017, several additional extreme weather events – including hurricanes – have impacted Puerto Rico, and future storms are inevitable.
CRediT authorship contribution statement
Deborah J. Watkins: Writing – original draft, Visualization, Supervision, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Héctor R. Torres Zayas: Writing – review & editing, Supervision, Project administration, Investigation, Data curation. Michael Welton: Writing – review & editing, Supervision, Project administration. Carmen M. Vélez Vega: Writing – review & editing, Project administration. Zaira Rosario Pabón: Writing – review & editing, Project administration. Luis D. Agosto Arroyo: Investigation, Data curation. Amber L. Cathey: Writing – review & editing, Visualization. Nancy R. Cardona Cordero: Writing – review & editing, Investigation. Akram Alshawabkeh: Project administration, Funding acquisition. José F. Cordero: Funding acquisition, Conceptualization. John D. Meeker: Writing – review & editing, Funding acquisition, Conceptualization.
Data availability statement
Data will be made available upon request.
Ethics and consent
This study (the “Hurricane Study”) was approved by the research and ethics committees at the University of Puerto Rico Medical Sciences Campus (B0630117, August 2, 2018), and the PROTECT parent study was approved by the University of Puerto Rico Medical Sciences Campus (A8570110, July 2, 2011), University of Michigan School of Public Health (HUM00037064, 01/28/2010), and Northeastern University (IRB# 10-03-03, 06/29/2010). All participants verbally provided their full informed consent prior to participation in the Hurricane Study and written informed consent prior to participation in the PROTECT study.
Funding
This work is supported by the National Institute of Environmental Health Sciences (NIEHS) grants R21ES029751, P42ES017198, P50ES026049, and UG/H3OD023251, EPA grant R836155, and National Institute on Minority Health and Health Disparities grants R21MD013709.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e39767.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
References
- 1.Robinson W.A. Climate change and extreme weather: a review focusing on the continental United States. J. Air Waste Manag. Assoc. 2021;71(10):1186–1209. doi: 10.1080/10962247.2021.1942319. 1995. [DOI] [PubMed] [Google Scholar]
- 2.Shenoy S., Gorinevsky D., Trenberth K.E., Chu S. Trends of extreme US weather events in the changing climate. Proc Natl Acad Sci U S A. 2022;119(47) doi: 10.1073/pnas.2207536119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandipati S., Abel D.E. Anticipated impacts of climate change on women's health: a background primer. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2023;160(2):394–399. doi: 10.1002/ijgo.14393. [DOI] [PubMed] [Google Scholar]
- 4.Barkin J.L., Philipsborn R.P., Curry C.L., et al. Climate change is an emerging threat to perinatal mental health. J. Am. Psychiatr. Nurses Assoc. 2022 doi: 10.1177/10783903221139831. Published online December 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veenema R.J., Hoepner L.A., Geer L.A. Climate change-related environmental exposures and perinatal and maternal health outcomes in the U.S. Int J Environ Res Public Health. 2023;20(3) doi: 10.3390/ijerph20031662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan W., Zlatnik M.G. Climate change and pregnancy: risks, mitigation, adaptation, and resilience. Obstet. Gynecol. Surv. 2023;78(4):223–236. doi: 10.1097/OGX.0000000000001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ha S. The changing climate and pregnancy health. Curr Environ Health Rep. 2022;9(2):263–275. doi: 10.1007/s40572-022-00345-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothschild J., Haase E. The mental health of women and climate change: direct neuropsychiatric impacts and associated psychological concerns. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2023;160(2):405–413. doi: 10.1002/ijgo.14479. [DOI] [PubMed] [Google Scholar]
- 9.Rothschild J., Haase E. Women's mental health and climate change Part II: socioeconomic stresses of climate change and eco-anxiety for women and their children. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2023;160(2):414–420. doi: 10.1002/ijgo.14514. [DOI] [PubMed] [Google Scholar]
- 10.USCB. Quick Facts: Puerto Rico. United States Census Bureau Accessed July 16, 2023. https://www.census.gov/quickfacts/fact/table/PR/PST045221.
- 11.Garza J.R., Perez E.A., Prelip M., et al. Occurrence and correlates of overweight and obesity among island Puerto Rican youth. Ethn. Dis. 2011;21(2):163–169. [PMC free article] [PubMed] [Google Scholar]
- 12.Perez C.M., Guzman M., Ortiz A.P., et al. Prevalence of the metabolic syndrome in san juan, Puerto Rico. Ethn. Dis. 2008;18(4):434–441. [PMC free article] [PubMed] [Google Scholar]
- 13.Ely D.M., Driscoll A.K. Infant mortality in the United States, 2020: data from the period linked birth/infant death file. 2022. https://www.cdc.gov/nchs/data/nvsr/nvsr71/nvsr71-05.pdf [PubMed]
- 14.Padilla I., Irizarry C., Steele K. Historical contamination of groundwater resources in the north coast karst aquifers of Puerto Rico. Rev Dimens. 2011;3:7–12. [PMC free article] [PubMed] [Google Scholar]
- 15.Guerra Velázquez G.R. Hurricane maría and public health in Puerto Rico: lessons learned to increase resiliency and prepare for future disasters. Ann Glob Health. 2022;88(1):82. doi: 10.5334/aogh.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watkins D.J., Torres Zayas H.R., Vélez-Vega C.M., et al. Investigating the impact of Hurricane Maria on an ongoing birth cohort in Puerto Rico. Popul. Environ. 2020;42(1):95–111. doi: 10.1007/s11111-020-00345-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lafarga Previdi I., Welton M., Díaz Rivera J., et al. The impact of natural disasters on maternal health: hurricanes Irma and maría in Puerto Rico. Child Basel Switz. 2022;9(7) doi: 10.3390/children9070940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Presley S.M., Abel M.T., Austin G.P., et al. Metal concentrations in schoolyard soils from New Orleans, Louisiana before and after hurricanes Katrina and Rita. Chemosphere. 2010;80(1):67–73. doi: 10.1016/j.chemosphere.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 19.Albering H.J., van Leusen S.M., Moonen E.J., Hoogewerff J.A., Kleinjans J.C. Human health risk assessment: a case study involving heavy metal soil contamination after the flooding of the river Meuse during the winter of 1993-1994. Environ. Health Perspect. 1999;107(1):37–43. doi: 10.1289/ehp.9910737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.USEPA UEPA National priorities list (NPL) sites - by state. 2023. https://www.epa.gov/superfund/national-priorities-list-npl-sites-state#PR
- 21.Lin Y., Sevillano-Rivera M., Jiang T., et al. Impact of hurricane Maria on drinking water quality in Puerto Rico. Environ. Sci. Technol. 2020;54(15):9495–9509. doi: 10.1021/acs.est.0c01655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin Y., Li G., Rivera M.S., et al. Long-term impact of Hurricane Maria on point-of-use drinking water quality in Puerto Rico and associated potential adverse health effects. Water Res. 2024;265 doi: 10.1016/j.watres.2024.122213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hornung R.W., Reed L.D. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 1990;5(1):46–51. [Google Scholar]
- 24.Cathey A., Ferguson K.K., McElrath T.F., et al. Distribution and predictors of urinary polycyclic aromatic hydrocarbon metabolites in two pregnancy cohort studies. Env Pollut. 2018;232:556–562. doi: 10.1016/j.envpol.2017.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onyemauwa F., Rappaport S.M., Sobus J.R., Gajdosova D., Wu R., Waidyanatha S. Using liquid chromatography-tandem mass spectrometry to quantify monohydroxylated metabolites of polycyclic aromatic hydrocarbons in urine. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2009;877(11–12):1117–1125. doi: 10.1016/j.jchromb.2009.02.067. [DOI] [PubMed] [Google Scholar]
- 26.Kim S.S., Meeker J.D., Carroll R., et al. Urinary trace metals individually and in mixtures in association with preterm birth. Environ. Int. 2018;121(Pt 1):582–590. doi: 10.1016/j.envint.2018.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye X., Kuklenyik Z., Needham L.L., Calafat A.M. Quantification of urinary conjugates of bisphenol A, 2,5-dichlorophenol, and 2-hydroxy-4-methoxybenzophenone in humans by online solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2005;383(4):638–644. doi: 10.1007/s00216-005-0019-4. [DOI] [PubMed] [Google Scholar]
- 28.Ye X., Bishop A.M., Reidy J.A., Needham L.L., Calafat A.M. Parabens as urinary biomarkers of exposure in humans. Environ. Health Perspect. 2006;114(12):1843–1846. doi: 10.1289/ehp.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva M.J., Samandar E., Preau J.L., Reidy J.A., Needham L.L., Calafat A.M. Quantification of 22 phthalate metabolites in human urine. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2007;860(1):106–112. doi: 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 30.Ashrap P., Watkins D.J., Mukherjee B., et al. Predictors of urinary and blood Metal(loid) concentrations among pregnant women in Northern Puerto Rico. Environ. Res. 2020;183 doi: 10.1016/j.envres.2020.109178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atsdr A. 1995. Toxicological Profile for Polycyclic Aromatic Hydrocarbons. [PubMed] [Google Scholar]
- 32.Rodriguez-Carmona Y., Ashrap P., Calafat A.M., et al. Determinants and characterization of exposure to phthalates, DEHTP and DINCH among pregnant women in the PROTECT birth cohort in Puerto Rico. J. Expo. Sci. Environ. Epidemiol. 2020;30(1):56–69. doi: 10.1038/s41370-019-0168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mandigo A.C., DiScenza D.J., Keimowitz A.R., Fitzgerald N. Chemical contamination of soils in the New York City area following Hurricane Sandy. Environ. Geochem. Health. 2016;38(5):1115–1124. doi: 10.1007/s10653-015-9776-y. [DOI] [PubMed] [Google Scholar]
- 34.Abel M.T., Presley S.M., Rainwater T.R., et al. Spatial and temporal evaluation of metal concentrations in soils and sediments from New Orleans, Louisiana, USA, following hurricanes Katrina and Rita. Environ. Toxicol. Chem. 2007;26(10):2108–2114. doi: 10.1897/06-595R.1. [DOI] [PubMed] [Google Scholar]
- 35.Cobb G.P., Abel M.T., Rainwater T.R., et al. Metal distributions in New Orleans following hurricanes Katrina and Rita: a continuation study. Environ. Sci. Technol. 2006;40(15):4571–4577. doi: 10.1021/es060041g. [DOI] [PubMed] [Google Scholar]
- 36.Bera G., Camargo K., Sericano J.L., et al. Baseline data for distribution of contaminants by natural disasters: results from a residential Houston neighborhood during Hurricane Harvey flooding. Heliyon. 2019;5(11) doi: 10.1016/j.heliyon.2019.e02860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han I., Whitworth K.W., Christensen B., et al. Heavy metal pollution of soils and risk assessment in Houston, Texas following Hurricane Harvey. Environ Pollut Barking Essex 1987. 2022;296 doi: 10.1016/j.envpol.2021.118717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samon S.M., Rohlman D., Tidwell L.G., Hoffman P.D., Oluyomi A.O., Anderson K.A. Associating increased chemical exposure to hurricane Harvey in a longitudinal panel using silicone wristbands. Int J Environ Res Public Health. 2022;19(11) doi: 10.3390/ijerph19116670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samon S.M., Rohlman D., Tidwell L., et al. Determinants of exposure to endocrine disruptors following hurricane Harvey. Environ. Res. 2023;217 doi: 10.1016/j.envres.2022.114867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available upon request.