Abstract
A dysregulated host response to infection resulting in life-threatening organ dysfunction defines the onset of sepsis. Unfortunately, sepsis is common, costly, and deadly. The Surviving Sepsis Campaign publishes regularly updated, evidence-informed, detection, and treatment guidelines culminating in time-sensitive care “bundles.” The goal of these bundles is to expedite sepsis recognition because it is widely held that early treatment is life-saving. Hospitals are mandated to publicly report their bundle compliance, and this will soon be tied to hospital reimbursement. For these reasons, hospitals are creating sepsis emergency response teams which are a form of a rapid response team consisting of dedicated medical professionals who evaluate patients with suspected sepsis and initiate therapy when appropriate. Evidence to date support sepsis emergency response teams as a mechanism to improve bundle compliance, and potentially, patient outcome. Nevertheless, some elements of bundled sepsis care are controversial (e.g., intravenous fluid administration) as some argue that mandated treatment precludes personalized care. Herein, we briefly describe general sepsis emergency response team structure, review evidence supporting sepsis emergency response teams to improve bundle compliance and patient outcome and report our unique experience incorporating point of care ultrasound—to guide intravenous fluid—into a nursing-led sepsis team. We propose that our sepsis emergency response team approach allays concern that sepsis care is either bundled or personalized. Instead, incorporating point of care ultrasound into a nursing-led sepsis emergency response team increases bundle compliance and individualizes care.
Keywords: Severe sepsis, septic shock, point-of-care ultrasound, wearable technology, sepsis response team, nursing-led, fluid resuscitation, review
Introduction
Sepsis is life-threatening organ dysfunction due to a dysregulated host response to infection. 1 Though the nomenclature has changed in recent years, herein “sepsis” refers to infection associated with organ dysfunction, similar to the former definition of “severe sepsis.” Sepsis and septic shock are common, highly mortal conditions. 2 Indeed, there were between 300 and 1000 cases of sepsis per 100,000 people in the United States (US) in the first decade of the 21st century, and that number is increasing 3 ; in-hospital mortality for sepsis ranges between 20% and 40%.4 –6 From a global perspective, approximately 1-in-5 deaths are attributed to sepsis. 7 Additionally, sepsis is costly. In the United States in 2013, sepsis care cost approximately $23 billion 8 ; unsurprisingly, sepsis is associated with a 75% longer inpatient stay. 9 Thus, it is widely accepted that prompt recognition and treatment of sepsis is imperative to reduce its human and financial burden.10 –12
Therapy for sepsis and septic shock continues to evolve. Since Rivers and colleagues published the landmark Early Goal Directed Therapy (EGDT) protocol in 2001, 13 the Surviving Sepsis Campaign (SSC) has published numerous guidelines on sepsis and septic shock management. 14 As a result of the 2012 SSC update, the New York State Department of Health (NYSDOH) mandated that all hospitals in New York State follow evidence-based protocols for identifying and treating sepsis and septic shock. 10 The protocols included a 3-h bundle consisting of: (1) blood cultures before antibiotics, (2) serum lactate level, and (3) broad-spectrum antibiotics, all completed within 3 h. Furthermore, these protocols also required a 6-h bundle that includes an intravenous (IV) bolus of 30 mL/kg of body weight of crystalloid in patients with hypotension or a serum lactate level of 4.0 mmol or more. 15 The 6-h bundle also included vasopressors for refractory hypotension, and repeat serum lactate within 6 h of protocol initiation. Despite mixed evidence regarding efficacy of these bundles,6,11 (especially with respect to IV fluid resuscitation), 10 the SSC has recently mandated 1-h completion of the aforementioned.16 –19 Furthermore, bundle compliance must be reported publicly by all hospitals in the United States and will be tied to reimbursement beginning in 2026 as a part of the Centers for Medicare and Medicaid Services (CMS) value-based care initiative. Given the clinical and financial importance of recognizing and treating sepsis expeditiously, interest in sepsis emergency response teams (SERTs) is growing. A SERT is similar to rapid or pulmonary embolism response team (i.e., RRT, PERT), however, focused on identifying and treating sepsis and septic shock.20,21
Over the last 2 decades, observational studies on the efficacy of SERTs have been published and reviewed. These studies often showed that SERT implementation associates with reduced sepsis mortality and, almost ubiquitously, increases guideline or bundle compliance. As outlined by both Funk et al. 22 as well as Bloos, 20 a SERT is characterized by its organization, trigger, response (including personnel and equipment) and quality improvement mechanism. Using this framework as a point-of-departure, we review and summarize basic elements of a SERT, give an overview of previously reported SERT investigations and describe our unique experience led by nurses trained in point-of-care ultrasound (POCUS). 23 We argue that nurses using POCUS can improve bundle compliance and individualize IV fluid therapy early in the care of sepsis and septic shock.
A brief overview of sepsis response team structure
Early identification of patients with sepsis and septic shock by a “sepsis emergency response team” (SERT) is believed to improve outcomes.20 –22,24 A SERT is defined as a form of a rapid response team (RRT) consisting of dedicated medical professionals who clinically evaluate patients suspected to have sepsis and initiate therapy when appropriate. 21 Fundamentally, for a SERT to be successful it should contain four basic parts22,25,26 (Table 1).
Table 1.
Basic SERT components.
| SERT component | Description |
|---|---|
| “Central nervous system” or administrative | Plans, obtains resources, educates, implements, and structures the SERT. This often begins by identifying, supporting and empowering local SERT champions |
| “Afferent limb” | Like a sensory nerve obtaining environmental data. This part of the SERT searches for and detects patients with sepsis or septic shock. The afferent limb uses clinical signs and symptoms that are, ideally, both sensitive and specific for identifying sepsis. Traditionally, early recognition of sepsis belonged to bedside clinicians. 22 Increasingly, the afferent limb is supplemented by electronic medical records that scan patient data for early warning.27,28 |
| “Efferent limb” | Includes mobile resources (personnel with defined competencies and specialized equipment) that arrive quickly, assess, and intervene early |
| “Quality assurance” or feedback | By measuring and monitoring intended SERT outcomes, adjustments can be made, iteratively, for process improvements to the afferent and efferent elements. This final SERT component is often part of the aforementioned “administrative” arm. 22 |
SERT: sepsis emergency response team.
Afferent arm
Although an exhaustive review of SERT triggers is beyond the scope of this manuscript, a brief explanation is warranted. Both Bloos and Uffen and colleagues have provided up-to-date overviews on SERT alerts.20,21 Usually, the goal of a screening tool is to be overly sensitive (i.e., minimize false negatives) while accepting diminished specificity (i.e., tolerate more false positives). The downside to decreased specificity is that too many false positive alerts can cause “alarm fatigue.”27 –30 In general, in the emergency department, the presence of at least two markers of the systemic inflammatory response syndrome (SIRS) has good sensitivity but variable or poor specificity for predicting life-threatening organ dysfunction due to infection. 21 This explains why the presence of SIRS has only a weak association with mortality.31,32 Another clinical score used to predict sepsis is a derivative of the SOFA score, the “quick” SOFA or qSOFA. 32 The ability of qSOFA to detect sepsis suffers from the “opposite” problem of the SIRS criteria. That is, qSOFA of at least two has a very good specificity (i.e., there are fewer false positives, which reduces alarm fatigue) and is a good predictor of death or ICU admission. 33 Unfortunately, qSOFA can have quite low sensitivity.34,35 In other words, a clinically significant proportion of patients in the ED who go on to develop life-threatening organ dysfunction do not have at least two qSOFA early in their care. Given that screening tests should minimize false negatives (i.e., have high sensitivity), qSOFA is a less-appealing trigger.36,37
Efferent arm
There is great heterogeneity in SERT composition. The efferent arm (i.e., the personnel and equipment brought to the patient) may be composed of trainees (e.g., residents or fellows), critical care physicians, nurses and nurse practitioners, infectious disease specialists, phlebotomists, lab runners, radiology technologists, respiratory therapists, pharmacists, and bed coordinators. Their equipment and resources include, but are not limited to antibiotics, fluids, venous access tools, vasoactive medications, respiratory support, 15 and portable ultrasound technology for hemodynamic assessment, as described below.
SERT implementation: Effects on guideline compliance and hospital mortality
As this was a literature review, approval by a research ethics board and patient consent were unnecessary. We conducted a PubMed search for SERT studies published through December 31, 2023. Additionally, we screened all the of the references of the investigations and reviews that we identified. Only full manuscripts (i.e., not abstracts) in the English language were considered further. We included investigations with a dedicated SERT (i.e., additional personnel with or without specialized equipment presenting to the patient’s bedside), and that reported the effect of SERT implementation on guideline (or bundle) compliance and/or in-hospital mortality compared to no SERT. If the study reported only single elements of contemporary SEP-1 bundle compliance (e.g., time to antibiotics, lactate draw, cultures, etc.), it was included.
SERTs are itemized below based on whether they were led by nurses or nurse practitioners, what the afferent and efferent arms were, and whether POCUS was used to individualize resuscitation. We identified 34 studies and 26 are summarized below. The reasons for excluding the eight investigations are elaborated in the supplementary material.38 –45 Changes in guideline or bundle compliance or mortality are listed based upon statistical significance (i.e., p < 0.05 or less), unless otherwise stated.
Table 2 shows heterogeneity among the studies. This is best illustrated by the variation in baseline mortality rate (i.e., mortality prior to implementation of SERT or mortality rate of non-SERT treated patients) which ranged from 4% to 68% (median 25%, IQR: 13%–37%). Differences in patient population and time may explain this dissimilarity. For example, the study with the highest baseline mortality rate studied patients between 2005 and 2007, required organ dysfunction for SERT activation and included inpatients, 46 while the study with the lowest baseline mortality rate took place between 2016 and 2018, could have included patients meeting only two SIRS criteria (i.e., without organ dysfunction), and was restricted to the ED (i.e., early in disease course). 67 The median mortality change was an 8% reduction (i.e., −8%; IQR: −5% to −18%), though not all of the studies reporting reduced mortality were statistically significant. Of the 26 studies in Table 2, 13 (50%) found a statistically significant reduction in mortality associated with SERT implementation. The remaining 13 studies (50%) either found no significant change (i.e., 9 studies) or did not report or perform a statistical evaluation (i.e., 2 studies reported mortality change without statistical analysis and two studies did not report mortality numbers). When comparing hospital mortality prior to SERT implementation to after SERT implementation for the 24 studies that reported mortality, we found a significant decrease in associated mortality by student t-test (Figure 1); all data had normal distribution by the Kolmogorov–Smirnov test.
Table 2.
Overview of included SERT studies.
| Study | RN or NP led? | SERT call-point | Afferent arm | Efferent arm | POCUS used | SSC guideline or bundle compliance | Hospital mortality |
|---|---|---|---|---|---|---|---|
| Giradis et al. 46 | No | Hospital wide | ⩾2 SIRS criteria plus organ dysfunction | ICU and infectious disease attending physicians | Not mentioned | Improved | Decreased |
| Schramm et al. 47 | No | ICU | ⩾2 SIRS criteria plus organ dysfunction | ICU physician, nurse, respiratory therapists, pharmacist, vascular access technician, portable radiology technician, and the medical ICU secretary | Not mentioned | Improved | Decreased |
| Shiramizo et al. 48 | No | Hospital wide | ⩾2 SIRS criteria plus organ dysfunction | ICU MD and managing nurse | Not mentioned | Improved | Decreased |
| LaRosa et al. 49 | No | Hospital wide | ⩾2 SIRS criteria plus organ dysfunction | ICU MD, ICU RN, respiratory therapist, and pharmacist | Not mentioned | Improved | Decreased |
| Berg et al. 50 | No | Hospital wide | ⩾2 SIRS criteria plus organ dysfunction | Trauma surgeon, trauma NP or PA, SICU RN, RT, anaesthesiologist, and ICU pharmacist | Not mentioned | Improved (MAP goal and CVP measurement only) | No change |
| Seoane et al. 51 | No | ED or floors | RRT activation on floor with ⩾2 SIRS criteria plus 2 organ failures or serum lactic acid ⩾4.0 | Experienced ICU nurse, respiratory therapist with backup from the ICU resident | Not mentioned | Improved | Not reported |
| Benson et al. 52 | Yes | Medical and surgical floors | ⩾2 SIRS criteria (however, HR ⩾ 120 bpm; RR ⩾ 26 bpm) | Nurse practitioner | Not mentioned | Improved | No change |
| Flynn et al. 53 | No | ED, floors, ICU | ⩾2 SIRS criteria + order set by provider | Pharmacists, nursing RRT, bed control, and materials management with a “sepsis cart” | Not mentioned | Improved | No change |
| Jones et al. 54 | Yes | Floors | ⩾4 on an “early sepsis screening tool” | NP | Not mentioned | Not reported | Decreased |
| Umscheid et al. 55 | No | Inpatient acute care services | Novel EWRS score of at least 4 | Covering physician (e.g., intern), the bedside RN, and rapid response coordinators | Not mentioned | Improved | No change |
| Grek et al. 56 | No | ED | ⩾2 SIRS criteria plus organ dysfunction | ICU attending and fellow or resident, NP/PA, nursing supervisor, and pharmacist | Not mentioned | Improved (no p-value reported) | Decreased |
| Hayden et al. 57 | No | ED | ⩾2 SIRS criteria plus organ dysfunction (SWAT-A) | ED attending, ED technician, additional ED RN, radiology technician, pharmacist, pulmonary fellow as needed | Not mentioned | Improved | No change |
| Arabi et al. 58 | No | ED | ⩾2 SIRS criteria plus organ dysfunction | ICU physician and sepsis-trained RN | Not mentioned | Improved | Decreased |
| Guirgis et al. 59 | No | Hospital wide | ⩾2 SIRS criteria plus new altered mental status, or a systolic blood pressure ⩽ 90 mm Hg, or a serum lactate level ⩾ 3 mmol/L; rising MEWS-SRS of at least 5 triggered “possible sepsis” | RRT nurse, clinical pharmacist, and a respiratory technician (for general floor patients) | Not mentioned | Not reported | Decreased |
| Maclay and Rephann 60 | Yes | Floors | qSOFA ⩾ 2 | Critical care charge RN, respiratory therapist, laboratory phlebotomist | Not mentioned | Not reported | Decreased (no statistical analysis) |
| Viale et al. 61 | No | ED | ⩾2 SIRS criteria plus organ dysfunction and ED consultation | A single infectious disease attending | Not mentioned | Improved | Decreased (14-day mortality). No change for 30-day mortality |
| Rosenqvist et al. 62 | No | ED | SBP < 90 mmHg, oxygen saturation < 90%, RR > 30, HR > 130 or unconscious state plus documented or history of fever | ED and infectious disease attending | Not mentioned | Improved | No change |
| Thursky et al. 63 | No | Oncology hospital without an ED | ⩾2 SIRS criteria, or hypotension with SBP < 100 mmHg with an infection | Physician | Not mentioned | Improved | Decreased |
| MacMillan et al. 64 | No | MICU | ⩾2 SIRS criteria | Critical care pharmacist, phlebotomist, and the supervising resident physician | Not mentioned | Improved | No change |
| Delawder et al. 65 | Interdisciplinary | ED | ⩾3 SIRS criteria, 1 being either temperature or WBC criteria | Unit coordinator, technician, pharmacist, RT, and primary ED physician | Not mentioned | Improved | Decreased (no statistical analysis) |
| Ferguson et al. 66 | Yes | ED and inpatient floor | ⩾2 SIRS criteria | Physician, NP, or physician assistant | Not mentioned | Improved | Decreased |
| Whitfield et al. 67 | No | ED | ⩾2 of: temp >100.4 or <96.8°F, HR > 90 beats per minute, RR > 20 breaths per minute, SBP < 90 mmHg, confusion | ED MD, ED triage and charge nurses, clinical pharmacist, phlebotomist, and house supervisor | Not mentioned | Improved | Decreased |
| Suliman et al. 68 | No | ED | ⩾2 SIRS criteria | ICU fellow or resident | Not mentioned | Improved | No change |
| Semanco et al. 69 | Yes | Acute care area | ⩾3 SIRS criteria or ⩾2 SIRS criteria plus organ dysfunction | 2 CCRNs | Not mentioned | Improved (no statistical analysis) | Not reported |
| Schinkel et al. 70 | No | ED | MEWS ⩾ 3 | On-call MD from: emergency medicine, internal medicine, and the admitting specialty | Not mentioned | Improved | No change |
| Simon et al. 71 | No | ED | ⩾2 SIRS criteria | Primary MD, NP, PA, nurse, resource RN and ED pharmacist | Not mentioned | Improved | Decreased |
RN: registered nurse; NP: nurse practitioner; POCUS; point of care ultrasound; SSC: surviving sepsis campaign; ICU: intensive care unit; SIRS: systemic inflammatory response syndrome; MD: medical doctor; RT: respiratory therapist; SICU: surgical intensive care unit; PA: physician assistant: MAP: mean arterial pressure; CVP: central venous pressure; ED: emergency department; HR: heart rate; RR: respiratory rate; bpm: beats or breaths per minute; EWRS: early warning and response system; SWAT: sepsis workup and treatment; RRT: rapid response team; MEWS-SRS: modified early warning signs-sepsis recognition score; SBP: systolic blood pressure; °F: degrees Fahrenheit; CCRN: critical care registered nurse.
Figure 1.
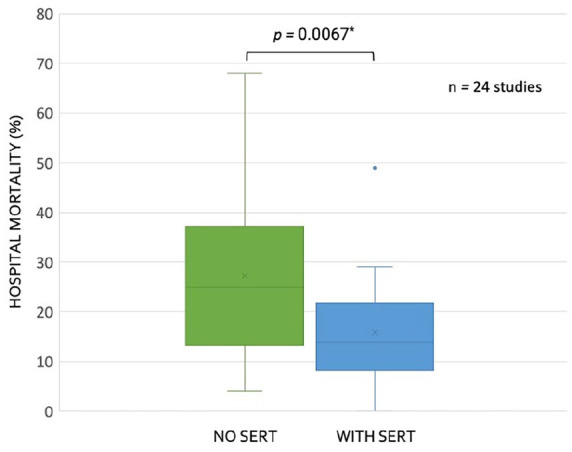
Box and whisker plots for reported mortality with and without sepsis emergency response team (SERT) implementation for studies (n = 24) reporting this data in Table 2.
Compliance with contemporary SSC guideline elements was ubiquitously improved by SERT as compared to no SERT. Because the definition of bundle compliance has changed over the last 20 years, we looked for whether SERT implementation associated with a statistically significant improvement in any or all of: antibiotic administration, blood culture or lactate collection, intravenous fluid provision or MAP goals for patients with sepsis or septic shock. 15 All studies that we located showed clinically and statistically significant improvement in at least one of these elements when SERT was implemented.
Limitations
Although these findings are noteworthy, causal inference cannot be drawn from the investigations reviewed in Table 2. As mentioned by Bloos, 20 before-and-after studies are quasi-experimental and at high risk of bias.20,72 Similar association between traditional RRT implementation and reduced mortality was observed, however, a randomized trial did not show significant benefit. 73 Furthermore, our review was not systematic, and we did not perform a funnel plot, so our search methodology as well as publication bias could partially account for these results. Thus, the effect on mortality we report should be viewed as hypothesis-generating and warrant more rigorous study.
Sepsis emergency response team structure: Our experience
Our SERT is composed of 14 RNs with specific training in POCUS and sepsis resuscitation. The SERT RNs work in collaboration with the primary care team and present the data collected including POCUS exam imaging and fluid responsiveness. The SERT is managed by the sepsis coordinator who also employs three RN data registrars. The data registrars audit each sepsis activation, afford feedback to care providers, and present the current data to the sepsis committee.
There is a total of three SERT RNs on duty per 12-h shift. All SERT nurses have worked in the ICU or ED for a minimum of 2 years prior to joining the team; their certifications include advanced cardiac and basic life support (i.e., ACLS, BLS), and pediatric advanced life support (PALS). SERTs may be initiated anywhere in the hospital as soon as the patient meets the internal activation criteria. The internal activation criteria utilized by our institution is defined as the presence of two or more SIRS with an end organ dysfunction and a known or suspected source of infection (Table 3).
Table 3.
SIRS criteria and end-organ dysfunction.
| SIRS criteria | Recognized organ dysfunctions |
|---|---|
| (1) Heart rate > 90 beats per min (2) Respiration rate > 20 breaths per min (3) Temperature >38 or <36°C (4) White blood cell (WBC) > 12,000 or <4,000 |
(1) Lactate >2 mmol/L (2) Acute altered level of consciousness (from patient’s baseline) (3) Systolic blood pressure < 90 mmHg or MAP < 65 mmHg (4) Increased oxygen requirements including intubation, CPAP/BiPAP or ⩾8 L/min of oxygen to maintain SpO2 ⩾90% (5) Consistent end tidal CO2 of ⩽34 mmHg or ⩾46 mmHg (6) Urine output < 30 mL/kg/h for 2 h, and acute changes in creatinine > 2 mg/dL (7) Platelets < 100,000/µL (8) Total bilirubin >2 mg/dL (9) INR > 1.5 |
Mmol: millimole; mmHg: millimeters of mercury; MAP: mean arterial pressure; CPAP and BiPAP: continuous positive and bilevel positive airway pressure, respectively; SpO2: oxygen saturation; CO2: carbon dioxide; L/min: liters per minute; mmHg: millimeters of mercury; mL/kg/h: milliliters per kilogram per hour; mg/dL: milligram per deciliter; mcL: microliter; INR: international normalized ratio.
Changes in vital signs or laboratory values can trigger Best Practice Alerts (BPAs) in the electronic medical record to help identify patients with severe sepsis while in the hospital. Once a BPA is triggered, the staff report findings to the primary care provider, who may then activate the SERT through the internal paging system. Once severe sepsis criteria are met, the SERT team is activated. The majority of activations begin in the ED. The first responders to the patient are the primary RN, the SERT RN, and pharmacist. If the patient is hemodynamically unstable, a physician will also be present. The SERT RN employs bedside ultrasound and a device to assess fluid responsiveness and/or fluid tolerance74 –76; this is discussed in more detail below. The primary physician, pharmacist, and the SERT RN then discuss the plan of care, including suspected source of infection with appropriate antibiotic coverage and orders are placed to ensure that the first hour bundle is met. If a fluid bolus is ordered, the SERT nurse will continue to recheck the patient post-bolus until resuscitation goals are met. Throughout the SERT activation the RN works actively with the primary team to ensure all bundle elements are completed, and the patient receives the highest quality of sepsis care.
Concerns and considerations
Though there are benefits to having dedicated care teams, one challenge faced by the SERT is that during times of high census, it can be difficult to get timely follow-up ultrasound exams—as described below—after a fluid bolus is given, because the SERT also responds to code strokes, cardiac arrests, and rapid responses. Therefore, human resource constraints are an obvious, ubiquitous, problem. Another challenge involved in SERT success is promoting effective team performance when caring for the acutely ill. Regular feedback from the “quality assurance” arm of the SERT in addition to peer-to-peer feedback after challenging cases improves team-based care over time; others note success with simulation training for improving teamwork. 77 Finally, the methods and technologies described below do require significant capital and human investment as we have previously described. 78 While long-term, patient-centered, outcome studies using our approach and technologies are lacking, we note that a similar argument can be made for many monitoring paradigms and tools in the ED and ICU (e.g., arterial lines, end-tidal carbon dioxide, pulse oximetry) where data are used to make physiologically guided decisions without obvious mortality benefit in a wide diversity of patients. Indeed, using point of care ultrasound to guide fluid resuscitation is a nascent area of research with a small and conflicted body of evidence to date.79 –81
Integrating point of care ultrasound into our SERT
In order to reduce mortality associated with sepsis, early recognition, source control, and antimicrobial therapy are essential.12,14 Nevertheless, there is controversy regarding SSC bundle compliance and patient outcome,6,11,16,17,19,21 especially with regards to IV fluid provision. 10 Criticism of mandated IV fluid administration to septic patients ultimately reduces to the tension between “guideline-based” and “personalized” medicine. That is to say, following “check boxes” disconnects the clinician from deliberating each patient’s unique presentation, pathology, physiological reserve, and therapy. 17 On the other hand, proponents of guidelines argue that bundles ensure basic standard-of-care for each patient, reduces practice variability and cognitive load, which can be important in fast-paced clinical scenarios. 82 To reconcile discord between guideline-informed and bespoke therapy, our nursing-led SERT employs POCUS for each patient to gauge the risk–benefit profile of IV fluid administration. 23
POCUS training
Initially, SERT trainees receive basic POCUS instruction, which includes operating specific ultrasound machines, differentiating phased, linear, and convex array probes, and determining their optimal use. The details of heart and lung ultrasound training are outlined in Table 4.
Table 4.
Basic and advanced POCUS competencies for SERT nurses.
| Basic POCUS training: Training in anatomical structures for probe placement to identify the inferior vena cava (IVC), cardiac structures, and lung landmarks. Competency in identifying and interpreting each of the below | ||
|---|---|---|
| IVC | Cardiac | Pulmonary |
| (1) Identification of the IVC and hepatic vein (2) Measurement of the IVC collapsibility utilizing M-mode (motion mode) (3) Measurement proficiency from either a subxiphoid or transverse view 2 cm distal from the hepatic vein |
(1) 4 windows: parasternal long and short axes, apical 4 chamber and subxiphoid views (2) Identification of chambers (RV, LV, RA, LA) and valves (TV, MV, PV, AV), and LVOT (3) Qualify LV function: normal, hyperdynamic/kinetic, hypodynamic/kinetic (4) Identify RV pressure or volume overload (D-sign or septal shift) (5) Identify pericardial pathology |
(1) Lung sliding (normal) (2) A-line profiles (normal) (3) B-lines (hyperechogenic vertical cones emanating from the pleural line, suggesting pulmonary edema in the correct clinical context) (4) Pleural spaces for effusions, loculations |
| Advanced POCUS training: Dedicated training in arterial and venous Doppler with advanced hemodynamic calculations. For example, quantification and qualification of right and left ventricular outflow tract velocity time integrals, estimating pulmonary artery systolic pressure (i.e., using the modified Bernoulli equation in the presence of tricuspid regurgitation), tricuspid annular plane systolic excursion, and VExUS | ||
LVOT: left ventricular outflow tract; VExUS: venous excess ultrasound score; SERT: sepsis emergency response team; POCUS: point of care ultrasound; IVC: inferior vena cava. RV and LV are right and left ventricles; RA and LA are right and left atria. TV, MV, PV, and AV are tricuspid, mitral, pulmonic, and aortic valves, respectively.
During all sepsis activations on their shift, the trainee performs a POCUS examination with an experienced rapid response RN preceptor. All images are saved and reviewed with a physician. Each trainee reviews 10 POCUS exams and clinical assessments with the lead physician in charge of ultrasound instruction. The trainee must demonstrate aptitude in order to pass training and certify competency. Afterward, SERT RNs maintain competency by exhibiting the aforementioned POCUS skills with the lead physician instructor yearly. To illustrate the effectiveness of our training program, we conducted a prospective observational study revealing that ED physicians agreed with SERT RN POCUS assessments in 99% of cases.78,83
Gauging the benefit–risk profile for IV fluids
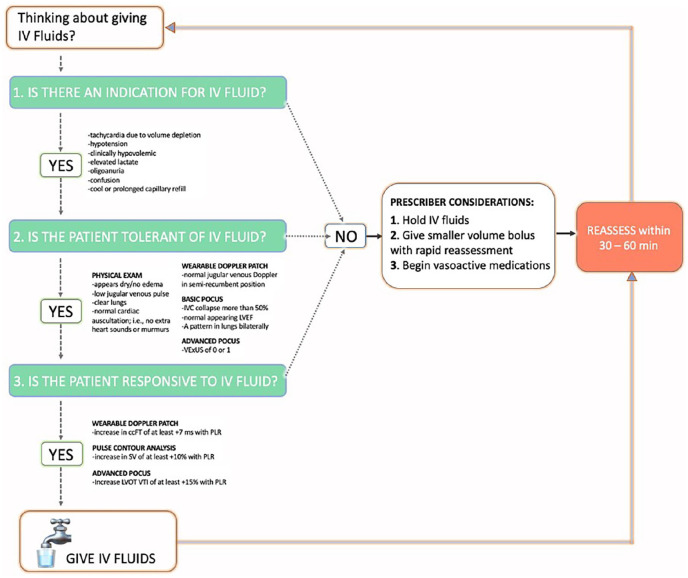
When our SERT responds to a patient, we perform a clinical examination supplemented by POCUS to gauge the benefit–risk profile for IV fluid. We consider IV fluid like a drug with organ-specific side-effects.84,85 We are particularly diligent about IV fluids because we see a high volume of septic patients with dialysis dependence, methamphetamine use, pulmonary hypertension, and congestive heart failure. As well, sepsis itself commonly associates with right ventricular dysfunction, which predicts mortality. 86 Given this, our approach to IV fluid is shown in Figure 2 and reduces to 3 basic questions:
Figure 2.
Overview of suggested fluid therapy pathway for our SERT.
(1) Does the patient have a clinical indication for IV fluid?
(2) Is the patient clinically tolerant to IV fluid?
(3) Is the patient fluid responsive?
If the answer to all three questions is “yes,” then the patient has a high benefit–risk ratio and IV fluids are encouraged. If the answer to any of these questions is “no,” then the provider is advised to reconsider fluids or give a smaller bolus volume with rapid re-evaluation (Figure 2). Accordingly, the more of these questions that are answered “no,” the lower the benefit-risk ratio for the individual patient. Brief elaboration of these concepts is considered in turn.
Question 1: Does the patient have a clinical indication for IV fluid?
While this question is obvious, it is sometimes forgotten. Like any drug, there should be a clinical need prior to provision. 84 If there is no need, then the therapy should be reconsidered. How do we assess need for IV fluid? Because the heart pumps blood volume to the organs and tissues, we look for signs and symptoms that this particular function (i.e., stroke volume or cardiac output) is impaired. A good list is the “10 signs of vitality” enumerated by Funk et al. 22 Of the 10, we pay particular attention to: unexplained tachycardia (i.e., not from pain, anxiety, fever, medication effect/withdrawal, etc.), hypotension (i.e., systolic blood pressure less than 90 mmHg or MAP less than 60–65 mmHg), tachypnea, diminished consciousness, oligoanuria (i.e., 30 mL/h for 5 h or <100 mL/4h—excluding renal failure), capillary refill longer than 3 s, 87 and elevated serum lactate. Though not specific, each of these markers could indicate reduced cardiac output and, therefore, oxygen delivery to organs and tissues. If any are present, then an indication for IV fluid (i.e., to augment stroke volume and cardiac output) is present; we then turn to assessing the patient’s safety profile for IV fluid, considered below in questions 2 and 3.
Question 2: Can the patient clinically tolerate IV fluids?
Within the sphere of critical care medicine, the entity of “fluid tolerance” has recently emerged.74,76,88 Nevertheless, this concept is a time-honored question grounded in the physical exam. Very simply, a patient can “tolerate” IV fluid volume if there are no signs of venous hypertension (i.e., venous congestion) or overt fluid overload. On examination, this is displayed by sunken eyes, reduced skin turgor, lack of underarm sweat, the absence of pre-tibial edema, jugular venous pulsations below the clavicle and normal cardiac and pulmonary auscultation (e.g., absence of murmurs, rubs, gallops, and bilateral crackles). 89 We supplement the clinical exam with both basic and advanced POCUS including data from a new wireless, wearable ultrasound Doppler.90 –95 Specifically, we evaluate for ultrasound signs of venous hypertension/congestion by looking for an inferior vena cava with less than 50% collapse on inspiration (i.e., a sign that the CVP is more than 10 mmHg), 96 reduced left ventricular (LV) ejection fraction (i.e., a LV high end-diastolic volume), bilateral B-lines on lung ultrasound (i.e., an ultrasonographic sign of pulmonary edema),97,98 or internal jugular venous Doppler signals consistent with high CVP in the semirecumbent position ascertained by the wearable Doppler device. 90 Furthermore, some of the SERT team is comfortable with advanced POCUS measures of venous hypertension, including the venous excess ultrasound score (VExUS). 99 We consider a VExUS score of 2 or more abnormal.88,100 We note that the LV assessment is a qualitative one and that integrating more advanced, objective measures such as mitral annular plane systolic excursion (MAPSE), e-point septal separation (EPSS), and fractional area changes are advanced measures that could reduce operator bias. If there are no physical or ultrasound exam findings concerning for venous hypertension or fluid overload, then the patient is considered “fluid tolerant,” and we proceed to question 3.
Question 3: Is the patient fluid responsive?
Fluid (or preload) responsiveness is a broad topic that has slowly gained traction over the last 20 years in the intensive care unit, operating room, and emergency department.101 –103 The definition of fluid responsiveness is a 10%–15% increase in stroke volume or cardiac output (i.e., SV or CO) following an IV fluid bolus. 103 Measuring the change in flow from the heart (i.e., SV or CO) is critical because other measures (including blood pressure) do not adequately predict whether or not IV fluid administered to a patient actually increases the SV or CO. In other words, if you need to know if the heart will increase flow, then flow needs to be measured! To make this more concrete, consider two patients who receive an IV fluid bolus; neither increase their mean arterial pressure (MAP). Patient A has a significant increase in SV or CO; this is quite common in sepsis (e.g., 50%–60% of patients who increase cardiac output do not increase their MAP).104,105 Patient A might benefit from more fluids and this clinical state would have been missed if using only MAP to guide therapy (e.g., the provider may have decided to use vasopressors prematurely). On the other hand, patient B has no change in SV or CO with IV fluids. Many patients early in sepsis care stop having a significant increase in SV or CO with IV fluids (e.g., 20%–30%).87,106 When these patients stop receiving IV fluid (i.e., cease trying “another bolus”), they have superior clinical outcomes and save the hospital money.107 –109
To test for fluid responsiveness, the SERT nurses perform a passive leg raise (PLR) maneuver110,111 with either SV monitoring via noninvasive or minimally invasive, uncalibrated pulse contour analysis, 112 or measured change in the corrected flow time of the carotid artery (ccFT) 113 measured by the wireless, wearable Doppler ultrasound system.114 –116 The change in the ccFT is measured automatically by the wearable, continuous wave, Doppler ultrasound that has a fixed, 60° insonation angle.94,95,117 The wireless, wearable ultrasound does not generate an image; its wide (>2 cm) ultrasound beam insonates the entire carotid artery, displays a continuous Doppler spectrogram, and automatically determines the largest change in ccFT during a 2-min PLR.75,93 Furthermore, some SERT RNs can measure the change in left ventricular outflow tract velocity time integral (LVOT VTI) 118 ; however, this is often too cumbersome to perform with the PLR, so is rarely done. Both the ccFT and LVOT VTI are Doppler ultrasound surrogates for SV. The PLR is a maneuver where a patient is moved from the semirecumbent position to supine with the legs elevated. This mobilizes 250–300 mL of blood from the abdomen and legs to the heart, acting like an internal blood bolus. 110 How the heart responds to this maneuver predicts how the heart will respond to IV fluids. 119
Concerns and considerations
It is not uncommon for patients to have signs, symptoms, or ultrasound findings concerning for fluid intolerance and/or fluid unresponsiveness early in their care.87,106,120 More specifically, and as shown in Figure 2, if a patient has findings that suggest that fluid intolerance (e.g., distended IVC, pulsatile jugular vein Doppler or B-lines, on lung ultrasound), and/or if the patient does not have a significant increase in SV or surrogate (e.g., ccFT, LVOT VTI) with PLR, the primary provider still makes the final decision regarding IV fluid. We do not treat this algorithm dogmatically or dichotomously, there is lots of physiological “gray zone,” and the response need not be “stop fluids entirely” or “keep giving fluids ceaselessly.” Patients can change rapidly in the ED. Therapies such as antibiotics, antipyretics, adrenergic agents, anxiolytics, analgesics, supplemental oxygenation, or assisted ventilation can all, theoretically, mediate how the heart responds to IV fluids within minutes-to-hours. This is because the inflammatory and sympathetic milieu change early in sepsis and following treatment. Thus, we stress re-assessment when IV fluid therapy is in doubt. Additionally, while ccFT and LVOT VTI change are clinically acceptable ways to detect fluid responsiveness with PLR,113,121,122 measurement error and expertise level can affect accuracy.123 –125 Lastly, in some cases of sepsis and septic shock, patients can be so “volume down” (e.g., if there is concomitant volume loss of approximately 1.5 L or more)126,127 that a PLR does not adequately bolus the heart and the patient will appear fluid unresponsive. In this scenario, after giving some crystalloid (i.e., roughly 20 mL/kg), the patient can become fluid responsive after the stressed venous volume is replenished.
Conclusions
We think it is likely that hospitals and health systems will form RRTs specifically for sepsis, that is, sepsis emergency response teams (SERTs). We believe this because there is good, and growing, evidence that urgently dedicating personnel and equipment to patients with sepsis increases compliance with time-sensitive treatment bundles laid out by the SSC. Because bundle compliance will soon be tied to hospital reimbursement, financial pressure will favor common-sense solutions. We believe strongly that rapid recognition and treatment of sepsis is expedited by SERTs and this likely improves patient outcome. Indeed, our review of SERT literature shows that SERT implementation associated with decreased sepsis mortality, though study methodology and variability preclude definitive conclusions. Perhaps the most controversial aspect of the SSC bundle is IV fluid provision. To address this, our SERT has trained RNs to implement a novel, ultrasound-supplemented treatment algorithm with the goal of expediting personalized sepsis resuscitation. This treatment pathway is not rigidly prescriptive; when there is uncertainty about IV fluid, the algorithm leans on the provider’s clinical judgement and suggests more frequent reassessment, just like any therapy in medicine. The algorithm is founded on using the physical exam and ultrasound to weigh the benefit–risk ratio for each patient. Incorporating “fluid responsiveness” testing in this algorithm will be considered “best practice” by the European Society of Intensive care medicine (ESICM) and has been shown to improve clinically meaningful outcomes in patients with sepsis and is cost-saving. In summary, a nursing-led SERT with competency in basic and advanced POCUS is feasible, novel and expedites individualized sepsis resuscitation.
Supplemental Material
Supplemental material, sj-docx-1-smo-10.1177_20503121241290378 for A nursing-led sepsis response team guiding resuscitation with point-of-care ultrasound: A review and model for improving bundle compliance while individualizing sepsis care by Jared Nunnally, So Mi Ko, Kristen Ugale, Tammy Lowe, Jacyln Bond, Jon-Emile S Kenny, Ramiz A Fargo and Korbin Haycock in SAGE Open Medicine
Acknowledgments
Not applicable
Footnotes
Author contributions: Conceptualization, TL, JB, RAF, and KH; methodology, JN, SMK, KU, and J-ESK; formal analysis, JN, SMK, KU, and J-ESK; resources, TL, JB, RAF, and KH; data curation, JN, SMK, KU, and J-ESK; writing—original draft preparation, JN, SMK, KU, and J-ESK; writing—review and editing, TL, JB, RAF, and KH; supervision and project administration, TL, JB, RAF, and KH. All authors have read and agreed to the published version of the manuscript.
Data availability statement: Data is available upon reasonable request.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J-ESK is the co-founder and chief medical officer of Flosonics Medical, the remaining authors declare no conflicts of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: Not applicable.
Institutional review board statement: Not applicable.
Informed consent statement: Not applicable.
ORCID iD: Jon-Emile S Kenny  https://orcid.org/0000-0002-3654-1146
https://orcid.org/0000-0002-3654-1146
Supplemental material: Supplemental material for this article is available online.
References
- 1. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Angus DC, Van der Poll T. Severe sepsis and septic shock. New Engl J Med 2013; 369: 840–851. [DOI] [PubMed] [Google Scholar]
- 3. Gaieski DF, Edwards JM, Kallan MJ, et al. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 2013; 41: 1167–1174. [DOI] [PubMed] [Google Scholar]
- 4. Kumar G, Kumar N, Taneja A, et al. Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest 2011; 140: 1223–1231. [DOI] [PubMed] [Google Scholar]
- 5. Kaukonen KM, Bailey M, Suzuki S, et al. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA 2014; 311: 1308–1316. [DOI] [PubMed] [Google Scholar]
- 6. Rhee C, Yu T, Wang R, et al. Association between implementation of the severe sepsis and septic shock early management bundle performance measure and outcomes in patients with suspected sepsis in US Hospitals. JAMA Netw Open 2021; 4: e2138596-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet 2020; 395: 200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Torio CM, Moore BJ. National inpatient hospital costs: the most expensive conditions by payer, 2013: statistical brief# 204. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville: MD: Agency for Healthcare Research and Quality (US); 2006. [PubMed] [Google Scholar]
- 9. Hall MJ, Williams SN, DeFrances CJ, et al. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. NCHS Data Brief 2011; 62: 1–8. [PubMed] [Google Scholar]
- 10. Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. New Engl J Med 2017; 376: 2235–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Townsend SR, Phillips GS, Duseja R, et al. Effects of compliance with the early management bundle (SEP-1) on mortality changes among medicare beneficiaries with sepsis: a propensity score matched cohort study. Chest 2022; 161: 392–406. [DOI] [PubMed] [Google Scholar]
- 12. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34: 1589–1596. [DOI] [PubMed] [Google Scholar]
- 13. Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. New Engl J Med 2001; 345: 1368–1377. [DOI] [PubMed] [Google Scholar]
- 14. Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med 2021; 47: 1181–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schorr C, Sebastien J, Dellinger R. Severe sepsis beyond the emergency department and ICU: targeting early identification and treatment on the hospital floor. Ann Update Intensive Care Emerg Med 2015; 2015: 557–569. [Google Scholar]
- 16. Klompas M, Rhee C. Has the medicare Sepsis Performance Measure (SEP-1) catalyzed better outcomes for patients with sepsis? Ann Intern Med 2021; 174(7): 1010–1011. [DOI] [PubMed] [Google Scholar]
- 17. Spiegel R, Farkas JD, Rola P, et al. The 2018 surviving sepsis campaign’s treatment bundle: when guidelines outpace the evidence supporting their use. Ann Emerg Med 2019; 73: 356–358. [DOI] [PubMed] [Google Scholar]
- 18. Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Intensive Care Med 2018; 44: 925–928. [DOI] [PubMed] [Google Scholar]
- 19. Rhee C, Gohil S, Klompas M. Regulatory mandates for sepsis care—reasons for caution. N Engl J Med 2014; 370: 1673–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bloos F. The importance of a hospital-dedicated sepsis response team. Expert Rev Anti Infect Ther 2020; 18: 1235–1243. [DOI] [PubMed] [Google Scholar]
- 21. Uffen JW, Oosterheert JJ, Schweitzer VA, et al. Interventions for rapid recognition and treatment of sepsis in the emergency department: a narrative review. Clin Microbiol Infect 2021; 27: 192–203. [DOI] [PubMed] [Google Scholar]
- 22. Funk D, Sebat F, Kumar A. A systems approach to the early recognition and rapid administration of best practice therapy in sepsis and septic shock. Curr Opin Crit Care 2009; 15: 301–307. [DOI] [PubMed] [Google Scholar]
- 23. Millington SJ, Wiskar K, Hobbs H, et al. Risks and benefits of fluid administration as assessed by ultrasound. Chest 2021; 160: 2196–2208. [DOI] [PubMed] [Google Scholar]
- 24. Ju T, Al-Mashat M, Rivas L, et al. Sepsis rapid response teams. Crit Care Clin 2018; 34: 253–258. [DOI] [PubMed] [Google Scholar]
- 25. Sebat F. Designing, implementing and enhancing a rapid response system. Mount Prospect, IL: Society of Critical Care Medicine, 2009, p. 217. [Google Scholar]
- 26. Sebat F, Johnson D, Musthafa AA, et al. A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients. Chest 2005; 127: 1729–1743. [DOI] [PubMed] [Google Scholar]
- 27. Harrison AM, Gajic O, Pickering BW, et al. Development and implementation of sepsis alert systems. Clin Chest Med 2016; 37: 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nelson JL, Smith BL, Jared JD, et al. Prospective trial of real-time electronic surveillance to expedite early care of severe sepsis. Ann Emerg Med 2011; 57: 500–504. [DOI] [PubMed] [Google Scholar]
- 29. Austrian JS, Jamin CT, Doty GR, et al. Impact of an emergency department electronic sepsis surveillance system on patient mortality and length of stay. J Am Med Inform Assoc 2018; 25: 523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Embi PJ, Leonard AC. Evaluating alert fatigue over time to EHR-based clinical trial alerts: findings from a randomized controlled study. J Am Med Inform Assoc 2012; 19: e145–e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315: 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raith EP, Udy AA, Bailey M, et al. Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA 2017; 317: 290–300. [DOI] [PubMed] [Google Scholar]
- 33. LeGuen M, Ballueer Y, McKay R, et al. Frequency and significance of qSOFA criteria during adult rapid response team reviews: a prospective cohort study. Resuscitation 2018; 122: 13–18. [DOI] [PubMed] [Google Scholar]
- 34. de Groot B, Stolwijk F, Warmerdam M, et al. The most commonly used disease severity scores are inappropriate for risk stratification of older emergency department sepsis patients: an observational multi-centre study. Scand J Trauma Resusc Emerg Med 2017; 25: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brink A, Alsma J, Verdonschot R, et al. Predicting mortality in patients with suspected sepsis at the Emergency Department; a retrospective cohort study comparing qSOFA, SIRS and National Early Warning Score. PLoS One 2019; 14: e0211133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fernando SM, Reardon PM, Rochwerg B, et al. Sepsis-3 septic shock criteria and associated mortality among infected hospitalized patients assessed by a rapid response team. Chest 2018; 154: 309–316. [DOI] [PubMed] [Google Scholar]
- 37. Boulos D, Shehabi Y, Moghaddas JA, et al. Predictive value of quick sepsis-related organ failure scores following sepsis-related medical emergency team calls: a retrospective cohort study. Anaesth Intensive Care 2017; 45: 688–694. [DOI] [PubMed] [Google Scholar]
- 38. Shapiro NI, Howell MD, Talmor D, et al. Implementation and outcomes of the Multiple Urgent Sepsis Therapies (MUST) protocol. Crit Care Med 2006; 34: 1025–1032. [DOI] [PubMed] [Google Scholar]
- 39. Castro R, Regueira T, Aguirre ML, et al. An evidence-based resuscitation algorithm applied from the emergency room to the ICU improves survival of severe septic shock. Minerva Anestesiol 2008; 74: 223–231. [PubMed] [Google Scholar]
- 40. Daniels R, Nutbeam T, McNamara G, et al. The sepsis six and the severe sepsis resuscitation bundle: a prospective observational cohort study. Emerg Med J 2011; 28: 507–512. [DOI] [PubMed] [Google Scholar]
- 41. Yarbrough N, Bloxam M, Priano J, et al. Pharmacist impact on sepsis bundle compliance through participation on an emergency department sepsis alert team. Am J Emerg Med 2019; 37: 762–763. [DOI] [PubMed] [Google Scholar]
- 42. Ludikhuize J, Marshall D, Devchand M, et al. Improving the management of medical emergency team calls due to suspected infections: a before-after study. Crit Care Resusc 2023; 25: 136–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sawyer AM, Deal EN, Labelle AJ, et al. Implementation of a real-time computerized sepsis alert in nonintensive care unit patients. Crit Care Med 2011; 39: 469–473. [DOI] [PubMed] [Google Scholar]
- 44. Focht A, Jones AE, Lowe TJ. Early goal-directed therapy: improving mortality and morbidity of sepsis in the emergency department. Jt Comm J Qual Patient Saf 2009; 35: 186–191. [DOI] [PubMed] [Google Scholar]
- 45. Lafon T, Baisse A, Karam HH, et al. Sepsis unit in the emergency department: impact on management and outcome of septic patients. Shock 2023; 60: 157–162. [DOI] [PubMed] [Google Scholar]
- 46. Girardis M, Rinaldi L, Donno L, et al. Effects on management and outcome of severe sepsis and septic shock patients admitted to the intensive care unit after implementation of a sepsis program: a pilot study. Crit Care 2009; 13: R143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schramm GE, Kashyap R, Mullon JJ, et al. Septic shock: a multidisciplinary response team and weekly feedback to clinicians improve the process of care and mortality. Crit Care Med 2011; 39: 252–258. [DOI] [PubMed] [Google Scholar]
- 48. Shiramizo SC, Marra AR, Durão MS, et al. Decreasing mortality in severe sepsis and septic shock patients by implementing a sepsis bundle in a hospital setting. PLoS One 2011; 6: e26790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Larosa JA, Ahmad N, Feinberg M, et al. The use of an early alert system to improve compliance with sepsis bundles and to assess impact on mortality. Crit Care Res Pract 2012; 2012: 980369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Berg GM, Vasquez DG, Hale LS, et al. Evaluation of process variations in noncompliance in the implementation of evidence-based sepsis care. J Healthc Qual 2013; 35: 60–69. [DOI] [PubMed] [Google Scholar]
- 51. Seoane L, Winterbottom F, Nash T, et al. Using quality improvement principles to improve the care of patients with severe sepsis and septic shock. Ochsner J 2013; 13: 359–366. [PMC free article] [PubMed] [Google Scholar]
- 52. Benson L, Hasenau S, O’Connor N, et al. The impact of a nurse practitioner rapid response team on systemic inflammatory response syndrome outcomes. Dimens Crit Care Nurs 2014; 33: 108–115. [DOI] [PubMed] [Google Scholar]
- 53. Flynn JD, McConeghy KW, Flannery AH, et al. Utilization of pharmacist responders as a component of a multidisciplinary sepsis bundle. Ann Pharmacother 2014; 48: 1145–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jones SL, Ashton CM, Kiehne L, et al. Reductions in sepsis mortality and costs after design and implementation of a nurse-based early recognition and response program. Jt Comm J Qual Patient Saf 2015; 41: 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Umscheid CA, Betesh J, VanZandbergen C, et al. Development, implementation, and impact of an automated early warning and response system for sepsis. J Hosp Med 2015; 10: 26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Grek A, Booth S, Festic E, et al. Sepsis and shock response team: impact of a multidisciplinary approach to implementing surviving sepsis campaign guidelines and surviving the process. Am J Med Qual 2017; 32: 500–507. [DOI] [PubMed] [Google Scholar]
- 57. Hayden GE, Tuuri RE, Scott R, et al. Triage sepsis alert and sepsis protocol lower times to fluids and antibiotics in the ED. Am J Emerg Med 2016; 34: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Arabi YM, Al-Dorzi HM, Alamry A, et al. The impact of a multifaceted intervention including sepsis electronic alert system and sepsis response team on the outcomes of patients with sepsis and septic shock. Ann Intensive Care 2017; 7: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Guirgis FW, Jones L, Esma R, et al. Managing sepsis: electronic recognition, rapid response teams, and standardized care save lives. J Crit Care 2017; 40: 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Maclay T, Rephann A. The Impact of early identification and a critical care-based sepsis response team on sepsis outcomes. Crit Care Nurse 2017; 37: 88–91. [DOI] [PubMed] [Google Scholar]
- 61. Viale P, Tedeschi S, Scudeller L, et al. Infectious diseases team for the early management of severe sepsis and septic shock in the emergency department. Clin Infect Dis 2017; 65: 1253–1259. [DOI] [PubMed] [Google Scholar]
- 62. Rosenqvist M, Fagerstrand E, Lanbeck P, et al. Sepsis Alert—a triage model that reduces time to antibiotics and length of hospital stay. Infect Dis (Lond) 2017; 49: 507–513. [DOI] [PubMed] [Google Scholar]
- 63. Thursky K, Lingaratnam S, Jayarajan J, et al. Implementation of a whole of hospital sepsis clinical pathway in a cancer hospital: impact on sepsis management, outcomes and costs. BMJ Open Qual 2018; 7: e000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. MacMillan A, Rudinsky D, Han G, et al. Multidisciplinary approach to improve sepsis outcomes. J Healthc Qual 2019; 41: 220–227. [DOI] [PubMed] [Google Scholar]
- 65. Delawder JM, Hulton L. An interdisciplinary code sepsis team to improve sepsis-bundle compliance: a quality improvement project. J Emerg Nurs 2020; 46: 91–98. [DOI] [PubMed] [Google Scholar]
- 66. Ferguson A, Coates DE, Osborn S, et al. Early, nurse-directed sepsis care. Am J Nurs 2019; 119: 52–58. [DOI] [PubMed] [Google Scholar]
- 67. Whitfield PL, Ratliff PD, Lockhart LL, et al. Implementation of an adult code sepsis protocol and its impact on SEP-1 core measure perfect score attainment in the ED. Am J Emerg Med 2020; 38: 879–882. [DOI] [PubMed] [Google Scholar]
- 68. Suliman S, Price J, Cahill M, et al. Bedside evaluation for early sepsis intervention: addition of a sepsis response team leads to improvement in sepsis bundle compliance. Crit Care Explor 2021; 3: e0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Semanco M, Wright S, Rich RL. Improving initial sepsis management through a nurse-driven rapid response team protocol. Crit Care Nurse 2022; 42: 51–57. [DOI] [PubMed] [Google Scholar]
- 70. Schinkel M, Holleman F, Vleghels R, et al. The impact of a sepsis performance improvement program in the emergency department: a before-after intervention study. Infection 2023; 51: 945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Simon EL, Truss K, Smalley CM, et al. Improved hospital mortality rates after the implementation of emergency department sepsis teams. Am J Emerg Med 2022; 51: 218–222. [DOI] [PubMed] [Google Scholar]
- 72. Grimshaw J, Campbell M, Eccles M, et al. Experimental and quasi-experimental designs for evaluating guideline implementation strategies. Fam Pract 2000; 17(Suppl 1): S11–S16. [DOI] [PubMed] [Google Scholar]
- 73. Hillman K, Chen J, Cretikos M, et al. Introduction of the medical emergency team (MET) system: a cluster-randomised controlled trial. Lancet 2005; 365: 2091–2097. [DOI] [PubMed] [Google Scholar]
- 74. Kenny J-ES. Assessing fluid intolerance with doppler ultrasonography: a physiological framework. Med Sci 2022; 10: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kenny J-ÉS, Prager R, Rola P, et al. Simultaneous venous-arterial doppler ultrasound during early fluid resuscitation to characterize a novel doppler starling curve: a prospective observational pilot study. J Intensive Care Med 2024; 9(7): 628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kattan E, Castro R, Miralles-Aguiar F, et al. The emerging concept of fluid tolerance: a position paper. J Crit Care 2022; 71: 154070. [DOI] [PubMed] [Google Scholar]
- 77. Colman N, Patera A, Hebbar KB. Promoting teamwork for rapid response teams through simulation training. J Contin Educ Nurs 2019; 50: 523–528. [DOI] [PubMed] [Google Scholar]
- 78. Kalam S, Selden N, Haycock K, et al. Evaluating the effect of nursing-performed point-of-care ultrasound on septic emergency department patients. Cureus 2023; 15: e40519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yu K, Zhang S, Chen N, et al. Critical care ultrasound goal-directed versus early goal-directed therapy in septic shock. Intensive Care Med 2022; 48: 121–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Li L, Ai Y, Wang X, et al. Effect of focused cardiopulmonary ultrasonography on clinical outcome of septic shock: a randomized study. J Int Med Res 2021; 49: 3000605211013176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Skurzak S, Gallo M, Lavezzo B, et al. Goal-directed therapy in sepsis strikes back. Intensive Care Med 2022; 48: 502–503. [DOI] [PubMed] [Google Scholar]
- 82. Townsend SR, Tefera L, Rivers EP. Evidence underpinning the centers for medicare and medicaid services’ severe sepsis and septic shock management bundle (SEP-1). Ann Intern Med 2018; 168: 609–610. [DOI] [PubMed] [Google Scholar]
- 83. Selden N, Skaggs H, Lowe T, et al. 338 assessing the utility of nursing-performed point-of-care ultrasound as a guide to fluid resuscitation of septic patients in the emergency department. Ann Emerg Med 2017; 70: S134. [Google Scholar]
- 84. Finfer S, Myburgh J, Bellomo R. Intravenous fluid therapy in critically ill adults. Nat Rev Nephrol 2018; 14: 541–557. [DOI] [PubMed] [Google Scholar]
- 85. Ladzinski AT, Thind GS, Siuba MT. Rational fluid resuscitation in sepsis for the hospitalist: a narrative review. Mayo Clin Proc 2021; 96: 2464–2473. [DOI] [PubMed] [Google Scholar]
- 86. Vallabhajosyula S, Shankar A, Vojjini R, et al. Impact of right ventricular dysfunction on short-term and long-term mortality in sepsis: a meta-analysis of 1373 patients. Chest 2021; 159: 2254–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hernández G, Ospina-Tascón GA, Damiani LP, et al. Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-day mortality among patients with septic shock: the ANDROMEDA-SHOCK randomized clinical trial. JAMA 2019; 321: 654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kenny J-E, Prager R, Rola P, et al. Unifying fluid responsiveness and tolerance with physiology: a dynamic interpretation of the diamond-forrester classification. Crit Care Explor 2023; 5(12): e1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. McGee S, Abernethy WB, Simel DL. Is this patient hypovolemic? JAMA 1999; 281: 1022–1029. [DOI] [PubMed] [Google Scholar]
- 90. Kenny J-ES, Prager R, Rola P, et al. The effect of gravity-induced preload change on the venous excess ultrasound (VExUS) score and internal jugular vein Doppler in healthy volunteers. Intensive Care Med Exp 2023; 11: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kenny JS, Gibbs SO, Johnston D, et al. Continuous venous-arterial doppler ultrasound during a preload challenge. J Vis Exp 2023; 191. DOI: 10.3791/64410. [DOI] [PubMed] [Google Scholar]
- 92. Kenny J-ÉS, Barjaktarevic I, Mackenzie DC, et al. Inferring the Frank–Starling curve from simultaneous venous and arterial doppler: measurements from a wireless, wearable ultrasound patch. Front Med Technol 2021; 3: 676995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kenny JS, Gibbs SO, Eibl JK, et al. Simultaneous venous-arterial Doppler during preload augmentation: illustrating the Doppler Starling curve. Ultrasound J 2023; 15: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kenny J-ÉS, Munding CE, Eibl JK, et al. A novel, hands-free ultrasound patch for continuous monitoring of quantitative Doppler in the carotid artery. Scient Rep 2021; 11: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kenny J-ES. Wearable ultrasound for continuous deep-tissue monitoring. Nat Biotechnol 2024; 42(3): 386–387. [DOI] [PubMed] [Google Scholar]
- 96. Kircher BJ, Himelman RB, Schiller NB. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol 1990; 66: 493–496. [DOI] [PubMed] [Google Scholar]
- 97. Lichtenstein DA. BLUE-protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill. Chest 2015; 147: 1659–1670. [DOI] [PubMed] [Google Scholar]
- 98. Lichtenstein DA. Lung ultrasound in the critically ill. Ann Intensive Care 2014; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Beaubien-Souligny W, Rola P, Haycock K, et al. Quantifying systemic congestion with point-of-care ultrasound: development of the venous excess ultrasound grading system. Ultrasound J 2020; 12: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Longino A, Martin K, Leyba K, et al. Correlation between the VExUS score and right atrial pressure: a pilot prospective observational study. Crit Care 2023; 27: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Monnet X, Teboul J-L. Passive leg raising: five rules, not a drop of fluid! Crit Care 2015; 19: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Monnet X, Shi R, Teboul J-L. Prediction of fluid responsiveness. What’s new? Ann Intensive Care 2022; 12: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Michard F, Teboul J-L. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest 2002; 121: 2000–2008. [DOI] [PubMed] [Google Scholar]
- 104. García MIM, González PG, Romero MG, et al. Effects of fluid administration on arterial load in septic shock patients. Intensive Care Med 2015; 41: 1247–1255. [DOI] [PubMed] [Google Scholar]
- 105. Magder S, Bafaqeeh F. The clinical role of central venous pressure measurements. J Intensive Care Med 2007; 22: 44–51. [DOI] [PubMed] [Google Scholar]
- 106. Kattan E, Ospina-Tascón GA, Teboul J-L, et al. Systematic assessment of fluid responsiveness during early septic shock resuscitation: secondary analysis of the ANDROMEDA-SHOCK trial. Crit Care 2020; 24: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Douglas IS, Alapat PM, Corl KA, et al. Fluid response evaluation in sepsis hypotension and shock: a randomized clinical trial. Chest 2020; 158: 1431–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Satterwhite L, Latham H. Fluid management in sepsis hypotension and septic shock: time to transition the conversation from fluid responsive to fluid refractory? Chest 2020; 158: 1319–1320. [DOI] [PubMed] [Google Scholar]
- 109. Latham HE, Bengtson CD, Satterwhite L, et al. Stroke volume guided resuscitation in severe sepsis and septic shock improves outcomes. J Crit Care 2017; 42: 42–46. [DOI] [PubMed] [Google Scholar]
- 110. Monnet X, Teboul J-L. Passive leg raising. Intensive Care Med 2008; 34: 659–663. [DOI] [PubMed] [Google Scholar]
- 111. Monnet X, Marik P, Teboul J-L. Passive leg raising for predicting fluid responsiveness: a systematic review and meta-analysis. Intensive Care Med 2016; 42: 1935–1947. [DOI] [PubMed] [Google Scholar]
- 112. Ameloot K, Palmers P-J, Malbrain ML. The accuracy of noninvasive cardiac output and pressure measurements with finger cuff: a concise review. Curr Opin Critical Care 2015; 21: 232–239. [DOI] [PubMed] [Google Scholar]
- 113. Barjaktarevic I, Toppen WE, Hu S, et al. Ultrasound assessment of the change in carotid corrected flow time in fluid responsiveness in undifferentiated shock. Crit Care Med 2018; 11: 1040–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kerrebijn I, Atwi S, Horner C, et al. Correlation between changing carotid artery corrected flow time and ascending aortic Doppler flow velocity. Br J Anaesth 2023; 131(6): e192–e195. [DOI] [PubMed] [Google Scholar]
- 115. Kenny J-ÉS, Barjaktarevic I, Mackenzie DC, et al. Carotid Doppler ultrasonography correlates with stroke volume in a human model of hypovolaemia and resuscitation: analysis of 48,570 cardiac cycles. Br J Anaesth 2021; 127: e60–e63. [DOI] [PubMed] [Google Scholar]
- 116. Kenny JS, Barjaktarevic I, Eibl AM, et al. Temporal concordance between pulse contour analysis, bioreactance and carotid doppler during rapid preload changes. PLoS One 2022; 17: e0265711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kenny J-ÉS, Munding CE, Eibl AM, et al. Wearable ultrasound and provocative hemodynamics: a view of the future. Crit Care 2022; 26: 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Blanco P. Rationale for using the velocity–time integral and the minute distance for assessing the stroke volume and cardiac output in point-of-care settings. Ultrasound J 2020; 12: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Bentzer P, Griesdale DE, Boyd J, et al. Will this hemodynamically unstable patient respond to a bolus of intravenous fluids? JAMA 2016; 316: 1298–1309. [DOI] [PubMed] [Google Scholar]
- 120. Muñoz F, Born P, Bruna M, et al. Coexistence of a fluid responsive state and venous congestion signals in critically ill patients: a multicenter observational proof-of-concept study. Crit Care 2024; 28: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Singla D, Gupta B, Varshney P, et al. Role of carotid corrected flow time and peak velocity variation in predicting fluid responsiveness: a systematic review and meta-analysis. Korean J Anesthesiol 2023; 76: 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jozwiak M, Mercado P, Teboul J-L, et al. What is the lowest change in cardiac output that transthoracic echocardiography can detect? Crit Care 2019; 23: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Shaikh F, Kenny JE, Awan O, et al. Measuring the accuracy of cardiac output using POCUS: the introduction of artificial intelligence into routine care. Ultrasound J 2022; 14: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Abbasi A, Azab N, Nayeemuddin M, et al. Change in carotid blood flow and carotid corrected flow time assessed by novice sonologists fails to determine fluid responsiveness in spontaneously breathing intensive care unit patients. Ultrasound Med Biol 2020; 46: 2659–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Bussmann BM, Sharma S, Mcgregor D, et al. Observational study in healthy volunteers to define interobserver reliability of ultrasound haemodynamic monitoring techniques performed by trainee doctors. Eur J Emerg Med 2019; 26: 217–223. [DOI] [PubMed] [Google Scholar]
- 126. Gelman S, Bigatello L. The physiologic basis for goal-directed hemodynamic and fluid therapy: the pivotal role of the venous circulation. Can J Anaesth 2018; 65: 294–308. [DOI] [PubMed] [Google Scholar]
- 127. Magder S. Volume and its relationship to cardiac output and venous return. Crit Care 2016; 20: 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-smo-10.1177_20503121241290378 for A nursing-led sepsis response team guiding resuscitation with point-of-care ultrasound: A review and model for improving bundle compliance while individualizing sepsis care by Jared Nunnally, So Mi Ko, Kristen Ugale, Tammy Lowe, Jacyln Bond, Jon-Emile S Kenny, Ramiz A Fargo and Korbin Haycock in SAGE Open Medicine