Abstract
Objective
Anlotinib demonstrated encouraging therapeutic activity as third-line treatment for patients with advanced non-small cell lung cancer (NSCLC). Programmed cell death protein 1 (PD-1) inhibitors also exhibited promising and durable response against previously-treated advanced NSCLC. Therefore, the present study aimed to determine the feasibility and safety of anlotinib plus PD-1 inhibitors for previously-treated NSCLC in clinical practice.
Methods
This retrospective study included 56 patients with advanced NSCLC treated with systemic treatment previously. Patients included were treated with anlotinib plus PD-1 inhibitors in clinical practice. Therapeutic outcomes of the patients were evaluated radiologically using target lesions, and the prognostic outcomes were generated by follow-up. Adverse reactions experienced throughout the treatment were documented and analyzed.
Results
Between August 2018 and November 2022, 56 patients with advanced NSCLC were eligible to participate in this study consecutively. Therapeutic outcomes resulted in an overall response rate of 28.6% [95% confidence interval (CI): 17.3%-42.2%] and a disease control rate of 91.1% (95% CI: 80.4%-97.0%). Furthermore, this combination regimen among the 56 patients yielded a median progression-free survival (PFS) of 6.5 months (95% CI: 4.81–8.19) and a median overall survival (OS) of 15.8 months (95% CI: 10.23–21.37), respectively. And the median duration of response (DoR) among patients who responded was 8.3 months (95% CI: 4.38–12.22). Additionally, adverse reactions of all grades throughout the treatment were observed in 50 patients (89.3%), and adverse reactions of grade ≥3 were detected in 23 patients (41.1%). Fatigue, hypertension, diarrhea, nausea, and vomiting were the most common adverse reactions. Association analysis between PFS and baseline characteristic subgroups indicated that ECOG score and number of metastatic lesions might be potential predictors of PFS in the exploratory analysis.
Conclusion
Anlotinib plus PD-1 inhibitors demonstrated a tolerable safety profile and encouraging therapeutic activity as subsequent-line therapy in patients with advanced NSCLC. This conclusion should be confirmed in prospective large-scale clinical trials subsequently.
Keywords: NSCLC, efficacy, tolerability, PD-1 inhibitors, anlotinib
Introduction
Lung cancer is the second most common solid tumor worldwide, and has the highest annual mortality rate. It was estimated that in 2018, there were approximately 2,093,876 new cases and 1,761,007 deaths globally.1 Approximately 815,000 new patients and 715,000 deaths of lung cancer were detected in China recently.2 Considerable patients were diagnosed with locally advanced or metastatic disease that surgical resection was not applicable.3 Noteworthily, recent years had witnessed that pathogenic genes of non-small cell lung cancer (NSCLC) were discovered and substantially investigated, and genotyping-based targeted drugs were developed accordingly.4 Targeted drug of EGFR-tyrosine kinase inhibitors (TKI) could result in a median survival of approximately 3 years among positive driver gene mutation population.5 However, approximately 50% of metastatic NSCLC cases were diagnosed with negative driver gene mutations, which meant that conventional chemotherapy might play a vital role as front-line therapy over the past several decades.
Encouragingly, therapeutic landscape of NSCLC were revolutionized by immunotherapy directed against programmed cell death protein 1 (PD-1), especially in NSCLC without driver gene mutations.6 It seemed that other immunotherapies (excluding immune checkpoint inhibitors) failed to provide sufficient evidence as the adjuvant setting among NSCLC patients with stages I to III.7 As a result, the application of PD-1 inhibitors was established as the standard regimen as the front-line treatment for metastatic NSCLC or in combination with chemotherapy in patients with previously-untreated advanced NSCLC, regardless of PD-L1 expression.8 To our knowledge, several PD-1/PD-L1 inhibitors had been approved the clinical administration for advanced NSCLC in China over the past few years. We noticed that pembrolizumab, nivolumab, sintilimab, camrelizumab, tislelizumab (PD-1 inhibitors) and durvalumab, atezolizumab (PD-L1 inhibitors) were all available immunotherapy-based regimens for advanced or metastatic NSCLC in China currently.9 In spite of the clinical trials regarding PD-1/PD-L1 inhibitors producing promising therapeutic outcomes, when patients experienced resistance to chemotherapy and immunotherapy, the overall response of immunotherapy monotherapy was only around 20% among unselected subjects, indicating the necessity to explore new therapeutic strategy that might broaden the clinical utility of anti-PD-1/PD-L1 inhibitors.10
According to Professor Folkman’s theory, angiogenesis was an essential process in tumor growth and metastasis. Prevention of angiogenesis might be used as a highly promising anticancer therapeutic approach, and studies on agents to hamper vessel formation in different cancers were conducted over the past decades.11 As a result, we noticed that bevacizumab or ramucirumab combined with chemotherapy or EGFR-TKI as first-line therapy for advanced or metastatic NSCLC demonstrated encouraging progression free survival (PFS) and promising overall survival (OS).12,13 Additionally, by inhibiting the key targets of angiogenesis,14 anlotinib was approved as the third-line standard treatment for advanced or metastatic NSCLC based on Phase III clinical trials.15 Anlotinib demonstrated superior antitumor activity as the third-line therapy among advanced or metastatic NSCLC with a dramatically prolonged prognosis (median PFS: 5.4 vs 1.4 months, P<0.001); median OS: 9.6 vs 6.3 months, P=0.002). Unfortunately, the objective response rate (ORR) of anlotinib monotherapy was disappointing in clinical practice (ORR=9.2%),16 highlighting the need to develop new combination strategies.17
Disorder in tumor vasculature was proven to be the potential mechanism of resistance to PD-1/PD-L1 inhibitors, resulting in immunosuppressive effects and compromising the clinical outcomes of immunotherapy.18,19 A previous meta-analysis highlighted that adding antiangiogenetic therapy to immunotherapy or chemotherapy might serve to identify the most appropriate candidate for this combination.20 Therefore, recent combination of PD-1/PD-L1 inhibitors with antiangiogenic targeted drugs had shown promising synergistic effects in cancer therapy.21 A recent exploratory mechanism study investigated the potential synergistic anti-tumor effect of anlotinib combined with immune checkpoint inhibitor in a lung cancer mouse model. Results showed that anlotinib potentiated the infiltration of innate immune cells, including natural killer cells and antigen-presenting cells, thereby contributing to a synergistic effect in vivo.22 Besides, we noticed that recent study explored the feasibility and toxicity of anlotinib plus PD-1 inhibitors for treatment refractory advanced or metastatic NSCLC.23 And the result indicated that PD-1 inhibitors plus anlotinib demonstrated promising anticancer efficacy for patients with advanced NSCLC clinically. However, the therapeutic outcomes of anlotinib plus PD-1 inhibitors for previously-treated advanced or metastatic NSCLC remained unclear. Therefore, the novelty of our study was to identify the efficacy and safety of anlotinib in combination with different PD-1 inhibitors in advanced or metastatic NSCLC, regardless of the gene mutation status.
Therefore, the present study aimed to investigate the therapeutic outcomes of anlotinib combined with PD-1 inhibitors in previously-treated advanced NSCLC in clinical practice.
Patients and Methods
Procedures and Eligibility Criteria
This retrospective study was performed in real-world setting. Subjects with advanced or metastatic NSCLC who had previously been exposed to systemic chemotherapy regimens at the Second Affiliated Hospital of Zhengzhou University between August 2018 and November 2022 were consecutively selected for this study. This study was conducted in accordance with clinical guidelines, approved by the Ethics Committee of the Second Affiliated Hospital of Zhengzhou University and in compliance with the Declaration of Helsinki. The requirement for informed consent was waived by the Ethics Committee due to the retrospective nature of the study, which involved the review of previously collected medical records without any direct patient involvement or intervention. Although patient consent was waived, all patient data were fully anonymized to ensure confidentiality throughout the study.
Although this was a retrospective study, the inclusion criteria were appropriately set to select suitable subjects. Main inclusion criteria were: (1) histologically or pathologically diagnosed recurrent, advanced, or metastatic NSCLC; (2) staging of IIIb or IV; (3) age ≥18 years; (4) performance status score of 0 to 2; (5) previous exposure to systemic therapy; (6) available tumor target lesion;24 (7) received PD-1 inhibitors combined with anlotinib treatment. Major exclusion criteria were as follows: (1) diagnosis of central lung squamous cell carcinoma; (2) active brain metastases with positive symptoms; (3) previous treatment with steroids or other immunosuppressive drugs; (4) diagnosis of more than one tumor or serious diseases that might lead to death; (5) efficacy assessment data was not available.
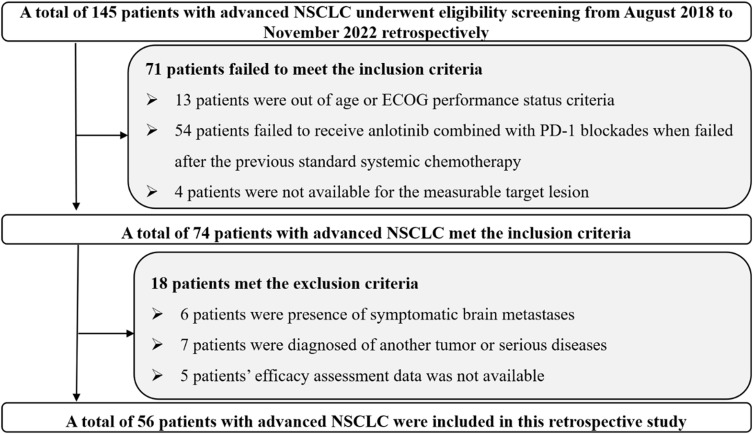
Study profile was shown in Figure 1. A total of 56 patients with advanced or metastatic NSCLC were included in this study. The primary endpoint of this study was PFS, other endpoints were ORR, disease control rate (DCR), OS, duration of response (DoR), and toxicity among the 56 patients treated with anlotinib plus PD-1 inhibitors.
Figure 1.
Flow chart of the feasibility and safety of anlotinib plus PD-1 inhibitors for previously treated advanced non-small cell lung cancer.
Therapeutic Regimens and Assessment Protocol
All 56 eligible patients were treated with PD-1 inhibitors plus anlotinib treatment. Anlotinib was administered once daily for 14 days orally and discontinued for 7 days, every 21 days was considered as one cycle. PD-1 inhibitors (any PD-1 monoclonal antibody licensed in China) included camrelizumab (Jiangsu Hengrui Medicine, China, 200 mg), tislelizumab (BeiGene, China, 200 mg), pembrolizumab (Merck & Co., USA; 200 mg), and nivolumab (Bristol-Myers Squibb, USA; 360 mg). All PD-1 inhibitors were administered intravenously on day 1 and every 21 days was considered as one cycle. Dose reduction of anlotinib to 10 mg or 8 mg was permitted, depending on potential adverse reactions.25
Efficacy assessment was performed using RECIST version 1.1. The response of target lesions was calculated using radiological scans (CT or MRI) when it was necessary in the clinic. ORR and DCR were determined with the outcomes of overall response assessed from the administration of anlotinib plus PD-1 inhibitors.26 Additionally, drug toxicity was assessed according to a previous study.25
Follow-Up Protocol
OS was the secondary endpoint, which was also analyzed in this study. Follow-up was performed by telephone during the study. The subjects were contacted once a month, and their living status was determined. The last follow-up date was May 10, 2024.
Genetic Profile Data Collection
Baseline genetic profile data, specifically EGFR and TP53 mutation status, were retrospectively collected from available clinical records. Unfortunately, due to the retrospective nature of this study, the pre-treatment tumor specimens of the patients were not available to perform the NGS analysis to conduct the genetic profile analysis. Therefore, we relied on pre-existing clinical records for genetic information. No new genetic testing was performed and baseline genetic profiling was limited to available mutation data. As a result, the total numbers of mutant genes were not available in this study.
Statistical Analysis
Regarding efficacy indicators, the definitions of ORR and DCR were adopted according to a previous study.21 All analyses were performed using SPSS version 25.0. Continuous and discrete variables were appropriately disposed. Survival endpoints were presented according to a previous study.27 DoR was the time from the first documentation of a complete response (CR) or partial response (PR) to the time of disease progression or death from any cause.28 The survival difference according to baseline characteristic subgroups was identified using Log rank test. Multivariate Cox regression analysis was adopted for PFS, and P<0.05, was deemed suggestive.
Results
Clinical Characteristics
Demographic and clinical characteristics of patients are presented in Table 1. Pathological staging suggested that most patients had stage IV (87.5%). A total of 45 patients were former smokers or smokers and 11 patients were nonsmokers. 37 and 19 patients had adenocarcinoma and squamous cell carcinoma, respectively. Interestingly, 11 subjects were treated with first-line therapy, and the other patients had received second-line or more treatment previously. Additionally, 9 patients had brain metastases, and 18 patients had previously undergone surgical resection. Notably, 19 (33.9%) patients had EGFR mutations. All 19 patients with EGFR mutations had sensitizing mutations and were previously treated with EGFR-TKIs. 51 patients in this study were available and 5 patients were not available for the TP53 mutation status. Of the 51 patients, TP53 positive mutation was detected in 28 patients, TP53 wild type was found in 23 patients. Four PD-1 inhibitors were used in this study. Camrelizumab, tislelizumab, pembrolizumab, and nivolumab were administered to 23, 16, 11, and 6 patients, respectively.
Table 1.
Baseline Characteristics of the 56 Patients with Advanced NSCLC
| Characteristics | Total Patients (N=56) | Percentage |
|---|---|---|
| Age (years) | ||
| Median (range) | 65 (21–81) | |
| ≥65 | 30 | 53.6% |
| <65 | 26 | 46.4% |
| ECOG performance status score | ||
| 0–1 | 25 | 44.6% |
| 2 | 31 | 55.4% |
| Gender | ||
| Male | 37 | 66.1% |
| Female | 19 | 33.9% |
| Pathological staging | ||
| IIIb | 7 | 12.5% |
| IV | 49 | 87.5% |
| Smoking status | ||
| Nonsmoker | 11 | 19.6% |
| Former smoker/smoker | 45 | 80.4% |
| Histological category | ||
| Adenocarcinoma | 37 | 66.1% |
| Squamous cell carcinoma | 19 | 33.9% |
| Lines of previous treatment | ||
| First-line | 11 | 19.6% |
| Second-line or more | 45 | 80.4% |
| Presence of brain metastases | ||
| Yes | 9 | 16.1% |
| No | 47 | 83.9% |
| History of surgical resection | ||
| Yes | 18 | 32.1% |
| No | 38 | 67.9% |
| EGFR mutation status | ||
| Positive | 19 | 33.9% |
| Negative | 37 | 66.1% |
| TP53 mutation status | ||
| Positive | 28 | 50.0% |
| Negative | 23 | 41.1% |
| NA | 5 | 8.9% |
| Number of metastatic lesions | ||
| ≤3 | 31 | 55.4% |
| >3 | 25 | 44.6% |
| Initial dosage of anlotinib (mg) | ||
| 12 | 35 | 62.5% |
| 10 | 21 | 37.5% |
| Immunotherapy | ||
| Camrelizumab | 23 | 41.1% |
| Tislelizumab | 16 | 28.6% |
| Pembrolizumab | 11 | 19.6% |
| Nivolumab | 6 | 10.7% |
Efficacy of the Combination Regimen
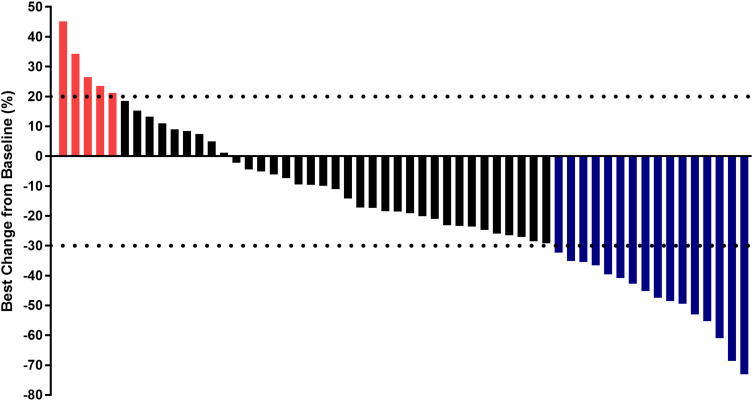
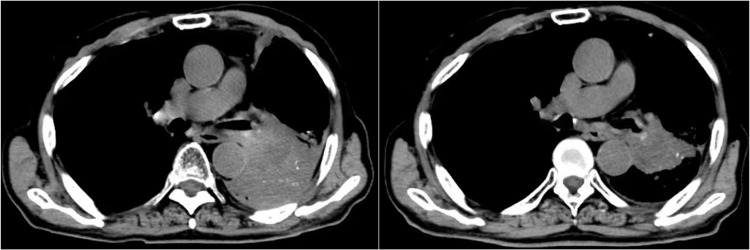
Radiological scans of the target lesions in the 56 subjects were collected and their responses were assessed individually. Response to the combination regimen showed that partial response (PR) was found in 16 cases, stable disease (SD) was detected in 35 subjects, and progressive disease (PD) was noted in 5 subjects, which resulted in an ORR of 28.6% (95% confidence interval [CI]: 17.3%-42.2%] and a DCR of 91.1% (95% CI: 80.4%-97.0%). The overall response of target lesions in the 56 patients was shown in Figure 2. The number of target lesions also changed significantly. A chest CT scan of the lung sites of one female patient before and after therapy with anlotinib plus pembrolizumab was shown in Figure 3. The target lesion was reduced dramatically after anlotinib plus pembrolizumab administration, and the pulmonary symptoms caused by the tumor were significantly relieved after combination treatment. The radiological results demonstrated that this patient could benefit from anlotinib plus pembrolizumab therapy.
Figure 2.
Waterfall plot for the response in target lesion of the 56 subjects with advanced non-small cell lung cancer who received anlotinib plus PD-1 inhibitors. (Blue columns mean PR, black columns mean SD and red column represents PD).
Figure 3.
CT scan results of the changes for target lesions in the lung site of one patient with advanced non-small cell lung cancer before and after the administration of anlotinib plus pembrolizumab.
Prognostic Outcomes of the Combination Regimen
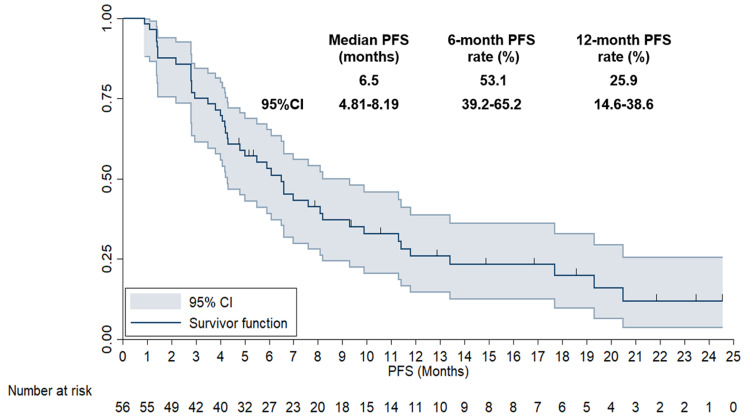
As described previously, the last follow-up date of the present study was May 10, 2024, which resulted in a median follow-up time of 14.5 months (follow-up duration: 0.9–33.5 months). In terms of PFS data, 43 disease progression or death events were detected, resulting in a PFS maturity rate of 76.8%. As illustrated in Figure 4, the median PFS of the 56 subjects was 6.5 months (95% CI: 4.81–8.19). Additionally, the 6-month and 12-month PFS rates were 53.1% (95% CI: 39.2%-65.2%) and 25.9% (95% CI: 14.6%-38.6%), respectively.
Figure 4.
Progression-Free Survival curve of the 56 patients with previously treated advanced non-small cell lung cancer who received anlotinib plus PD-1 inhibitors.
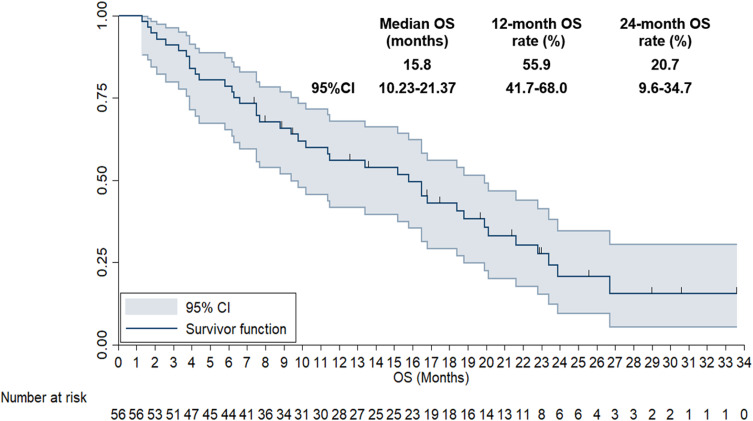
Given that the follow-up period was relatively adequate, long-term survival data were also available. Accordingly, 39 deaths were found at May 10, 2024, yielding a maturity rate of 69.6% for the OS data. As shown in Figure 5, the median OS of the 56 patients with advanced or metastatic NSCLC treated with anlotinib plus PD-1 inhibitors was 15.8 months (95% CI: 10.23–21.37). The 12-month and 24-month OS rates were 55.9% (95% CI: 41.7%-68.0%) and 20.7% (95% CI: 9.6%-34.7%), respectively.
Figure 5.
Overall Survival curve of the 56 patients with previously treated advanced non-small cell lung cancer who received anlotinib plus PD-1 inhibitors.
The univariate analysis of PFS and baseline characteristics were presented in Table 2. A considerable number of patients might benefit from anlotinib plus PD-1 inhibitor therapy. However, performance status and tumor burden were positively correlated with PFS in the univariate analysis as illustrated in Table 2, which indicated that patients with ECOG performance status of 0–1 score conferred a significantly longer PFS than those with 2 score (median PFS: 7.6 vs 4.8 months, P=0.009) and patients with lower tumor burden faced superior PFS than those with higher tumor burden (median PFS: 7.0 vs 5.0 months, P=0.018). Notably, patients who received anlotinib (12 mg) showed a trend for longer PFS than those who received 10 mg anlotinib, although no statistically significant difference was observed (median PFS: 7.0 vs 5.9 months, P=0.117, respectively). Similarly, it seemed that patients with TP53 positive mutation had a trend for superior PFS compared with the rest patients (median PFS: 8.2 months vs 5.5 months). However, the difference was not statistically significant (P=0.216). Subsequently, the characteristics that correlated with PFS were included in the multivariate Cox analysis. As illustrated in Table 2, multivariate Cox analysis results suggested that both number of metastatic lesions (HR=0.72, P=0.026) and ECOG performance status (HR=0.64, P=0.019) were independent factors for PFS.
Table 2.
Association Between PFS of the 56 Patients with Advanced NSCLC and Baseline Characteristic Subgroups in Univariate Analysis and Multivariate Cox Analysis
| Characteristics | Median PFS (95% CI) |
P (Univariate analysis) |
Multivariate analysis | |
|---|---|---|---|---|
| HR (95% CI) | P | |||
|
Age <65 ≥65 |
6.5 (4.75–8.25) 7.0 (5.12–8.88) |
0.617 |
||
|
ECOG performance status score 0–1 2 |
7.6 (5.36–9.84) 4.8 (3.68–5.92) |
0.009 |
0.64 (0.31–0.88) |
0.019 |
|
Gender Male Female |
6.1 (4.92–7.28) 6.5 (4.57–8.43) |
0.536 |
||
|
Pathological staging IIIb IV |
6.6 (4.75–8.45) 6.1 (4.96–7.24) |
0.512 |
||
|
Smoking status Nonsmoker Former smoker/smoker |
7.0 (5.37–8.63) 6.1 (4.72–7.48) |
0.428 |
||
|
Histological category Adenocarcinoma Squamous cell carcinoma |
6.6 (4.71–8.49) 6.1 (4.89–7.31) |
0.531 |
||
|
Lines of previous treatment First-line Second-line or more |
6.5 (4.92–8.08) 7.0 (5.37–8.63) |
0.613 |
||
|
Presence of brain metastases Yas No |
5.9 (4.67–7.13) 6.6 (4.91–8.29) |
0.339 |
||
|
History of surgical resection Yes No |
7.0 (5.35–8.65) 6.1 (4.93–7.27) |
0.311 |
||
|
EGFR mutation status Positive Negative |
6.1 (4.78–7.42) 6.6 (5.09–8.11) |
0.469 |
||
|
TP53 mutation status Mutation group Rest group |
8.2 (3.21–13.19) 5.5 (3.17–7.84) |
0.216 |
||
|
Number of metastatic lesions ≤3 >3 |
7.0 (5.08–8.92) 5.0 (3.83–6.17) |
0.018 |
0.72 (0.41–0.92) |
0.026 |
|
Initial dosage of anlotinib (mg) 12 10 |
7.0 (5.67–8.33) 5.9 (4.76–7.04) |
0.117 |
||
|
Immunotherapy Camrelizumab Tislelizumab Pembrolizumab Nivolumab |
6.1 (5.04–7.16) 7.0 (5.39–8.61) 6.5 (4.93–8.07) 6.6 (5.21–7.99) |
0.475 |
||
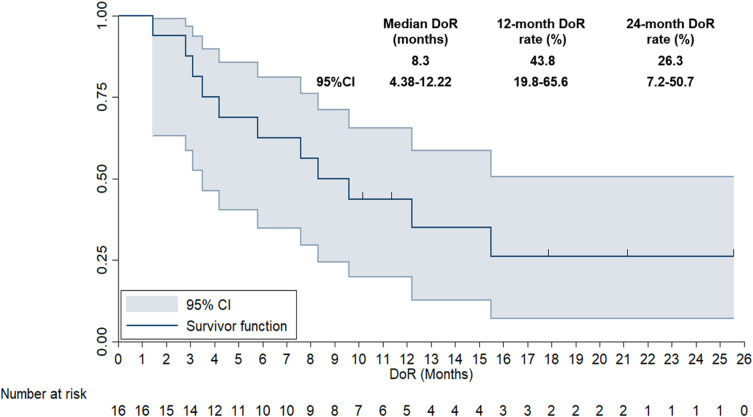
The DoR of 16 patients who achieved CR or PR was also analyzed, as illustrated in Figure 6. The median DoR of the 16 patients with advanced NSCLC was 8.3 months (95% CI: 4.38–12.22). The 12-month and 24-month DOR rates were 43.8% (95% CI: 19.8%-65.6%) and 26.3% (95% CI: 7.2%-50.7%), respectively.
Figure 6.
Duration of response of the 16 patients with previously treated advanced non-small cell lung cancer who achieved PR when received anlotinib plus PD-1 inhibitors.
Adverse Reactions of the Combination Regimen
As described previously, adverse reactions with the maximum grade among the 56 advanced or metastatic NSCLC patients observed throughout treatment with anlotinib plus PD-1 inhibitors were recorded. All toxicities, regardless of attribution, were detected in 50 patients (89.3%). Notably, this study failed to find any unexpected toxicity, and no deaths attributed to drug safety were observed throughout the combination treatment. Grade 3–4 adverse reactions were found in 23 patients (41.1%).
Furthermore, as shown in Table 3, the detailed safety profiles mentioned in this study included fatigue (64.3%), hypertension (55.4%), diarrhea (51.8%), nausea and vomiting (44.6%), rash (32.1%), hand-foot syndrome (26.8%), hepatotoxicity (21.4%), proteinuria (16.1%), pneumonia (12.5%), hemorrhage (10.7%), RCCEP (8.9%), and myelosuppression (8.9%). Furthermore, grade 3–4 toxicity included hypertension (16.1%), fatigue (8.9%), hepatotoxicity (7.1%), diarrhea (5.4%), nausea and vomiting (5.4%), rash (3.6%), hand-foot syndrome (3.6%), proteinuria (1.8%), and hematological toxicity (1.8%). From an objective view, the safety profile of 56 patients with advanced NSCLC treated with anlotinib plus PD-1 inhibitors was expected.
Table 3.
Adverse Reactions of the 56 Patients with Previous Chemotherapy Resistance Advanced or Metastatic NSCLC
| Adverse reactions | Total (N, %) | Grade 1–2 (N, %) | Grade 3–4 (N, %) |
|---|---|---|---|
| Adverse reactions of any grade | 50 (89.3) | 23 (41.1) | |
| Fatigue | 36 (64.3) | 31 (55.4) | 5 (8.9) |
| Hypertension | 31 (55.4) | 22 (39.3) | 9 (16.1) |
| Diarrhea | 29 (51.8) | 26 (46.4) | 3 (5.4) |
| Nausea and vomiting | 25 (44.6) | 22 (39.3) | 3 (5.4) |
| Rash | 18 (32.1) | 16 (28.6) | 2 (3.6) |
| Hand-foot syndrome | 15 (26.8) | 13 (23.2) | 2 (3.6) |
| Hepatotoxicity | 12 (21.4) | 8 (14.3) | 4 (7.1) |
| Proteinuria | 9 (16.1) | 8 (14.3) | 1 (1.8) |
| Pneumonia | 7 (12.5) | 6 (10.7) | 1 (1.8) |
| Hemorrhage | 6 (10.7) | 6 (10.7) | 0 (0.0) |
| RCCEP | 5 (8.9) | 5 (8.9) | 0 (0.0) |
| Myelosuppression | 5 (8.9) | 4 (7.1) | 1 (1.8) |
Discussion
In our opinion, this study provides real-world medical evidence regarding the feasibility and toxicity of combination of anlotinib and PD-1 inhibitors therapy in patients with previously-treated advanced NSCLC. Collectively, anlotinib plus PD-1 inhibitors regimen may be a potentially efficacious and encouraging therapeutic option for patients with previously-treated advanced NSCLC in clinical practice.
In spite of the substantial progress that immunotherapy and driver gene targeted drugs dramatically enhanced the survival of advanced or metastatic NSCLC, considerable patients might not benefit from the treatment ultimately with superior performance status that were able to receive the following treatment.29 Unfortunately, therapeutic outcomes in subsequent-line treatment of metastatic NSCLC remained disappointing.30 Currently, both anlotinib and PD-1/PD-L1 inhibitors were useful regimens as subsequent-line options for advanced or metastatic NSCLC clinically.31 Given that immunotherapy combined with systemic chemotherapy was confirmed to be the standard treatment as front-line for advanced or metastatic NSCLC currently, considerable patients might administer PD-1/PD-L1-based regimen in first-line setting, resulting in the relatively limited therapeutic value of immunotherapy as subsequent-line therapy.32 Therefore, anlotinib single agent might be an encouraging therapeutic regimen as a third-line option for treatment-refractory NSCLC in China based on the ALTER0303 clinical trial.15 However, similar as the clinical manifestation of PD-1 inhibitors (ORR<20%, median PFS <3 months),33 the response of anlotinib single agent was also dismal in clinical practice (ORR=9.2%). Further efforts were needed to improve the therapeutic outcomes of anlotinib as a single agent.
The patients with advanced NSCLC included in this study received at least first-line chemotherapy, indicating that the combination administration of anlotinib plus PD-1 inhibitors was reasonable, given that tislelizumab and pembrolizumab had the indications of second-line therapy for advanced or metastatic NSCLC. Overall, ORR among the 56 advanced or metastatic NSCLC patients who used anlotinib plus PD-1 inhibitors was 28.6%, DCR was 91.1%, and the median PFS was 6.5 months, which seemed to be better than the response and prognosis of patients with anlotinib single-agent arm in the ALTER0303 clinical trial numerically.15 The efficacy and PFS results were encouraging response, which was consistent with the results of a recent study regarding anlotinib combined with PD-1 inhibitors for advanced or metastatic NSCLC.18,23 In addition, we also observed another trial initiated by Zhang and colleagues compared PD-1 inhibitors plus anlotinib versus anlotinib alone for NSCLC patients.31 A total of 139 patients were assigned to receive PD-1 inhibitors plus anlotinib and anlotinib monotherapy, which yielded an ORR of 20.5% vs 18.2%, respectively. Additionally, the PD-1 inhibitor plus anlotinib group had significantly improved PFS compared to the anlotinib monotherapy group. Additionally, the previous trial recruited predominantly Chinese patients with advanced or metastatic NSCLC and compared the feasibility and safety of nivolumab and docetaxel in the second-line setting.34 Obviously, patients included in the CheckMate-078 trial were similar to those in our study, and after a 25.9 months follow-up, nivolumab yielded a durable response and a median OS of 11.9 and a 2-year OS rate of 28%, in line with the outcomes of our study numerically in this indirect comparison. To the best of our knowledge, the hyper-angiogenic nature might compromise the therapeutic outcomes of immunotherapy. The abnormal vascular structure of tumors caused by the overexpression of vascular growth factors might attenuate immune cell infiltration by forming a selective immune cell barrier or by suppressing immune cell function via aggravating the hypoxic microenvironment to maintain tumor growth and metastasis.35 Consequently, all these studies indicated that the therapeutic option of anlotinib combined with PD-1 inhibitors might play a synergistic antitumor action in clinical practice.
Notably, the Cox regression analysis results suggested that most subjects might benefit from the administration of anlotinib plus PD-1 inhibitors, which was consistent with a previous study. Similarly, a previous work chose 67 subjects to identify the efficacy analysis.18 And the univariate association analysis exhibited that only number of metastatic sites failed to benefit from anlotinib joint with PD-1 inhibitors treatment, suggesting efficacy of anlotinib plus PD-1 inhibitors was steady. Interestingly, we also found that both performance status and metastatic lesion status might predict PFS in the univariate analysis. It seemed that patients with an ECOG performance status of 2 and metastatic lesions of >3 failed to achieve better outcomes with anlotinib plus PD-1 inhibitors administration, similar to the previous study.26 Additionally, a higher tumor burden was also associated with worse PFS, which was in line with the results of the Cox multivariate analysis in ALTER0303 that patients with metastatic lesions of >3 predicted inferior prognosis.15 It seemed that patients with metastatic lesions of >3 had a higher tumor burden and tended to be correlated with inferior clinical outcomes.36 Although our study found significant associations between the ECOG score and number of metastatic lesions with PFS, these results should be interpreted with caution due to the lack of comprehensive genetic profiling analysis. Genetic factors, such as tumor mutation burden, which were not analyzed in this study thoroughly, might further influence the efficacy of anlotinib and PD-1 inhibitors. Interestingly, the preliminary genetic profile in our study highlighted that patient with TP53 positive mutation exhibited a trend toward superior PFS compared with the rest patients (median PFS: 8.2 months vs 5.5 months), although the difference was not statistically significant (P=0.216). Future prospective studies incorporating genetic testing were necessary to confirm these associations and ensure accurate prediction of the treatment outcomes. Interestingly, a previous study demonstrated that genetic background was one of the main factors affecting the response of cancers, including NSCLC,37 and 19 patients (33.9%) with EGFR positive mutations were included in our study, which meant that the genetic backgrounds of the subjects included in our study were different and heterogeneous. The results indicated that EGFR-positive mutations that accounted for 33.9% of the patients in our study might benefit from anlotinib plus PD-1 inhibitors therapy. In our opinion, recent work had indicated that the efficacy of PD-1/PD-L1 inhibitors monotherapy was disappointing among patients with EGFR mutation.38 Patients with EGFR positive mutation might not benefit from PD-1/PD-L1 monotherapy. Notably, the results from Impower150 suggested that EGFR-positive mutations might improve with the administration of atezolizumab (PD-L1 inhibitors) and bevacizumab plus chemotherapy compared with those who received bevacizumab plus chemotherapy.39 Final exploratory analysis in the Impower150 trial among patient subgroups with EGFR mutations highlighted that only patients with sensitive EGFR mutations might benefit from atezolizumab and bevacizumab plus chemotherapy treatment,40 which was also consistent with our study that all 19 patients with EGFR mutations were sensitive mutations and treated with EGFR-TKIs previously. Consequently, anlotinib combined with PD-1 inhibitors might be an effective therapeutic strategy for patients with EGFR-positive mutations. Additionally, we noticed that patients with brain metastasis seemed to benefit from the combination of anlotinib and PD-1 inhibitors treatment. To our knowledge, anlotinib has antiangiogenic properties and antitumor activity through JAK2/STAT3/VEGFA signaling in NSCLC cells, as indicated by previous work.41 As a result, anlotinib combined with PD-1 inhibitors should be treated as a promising therapeutic option for patients with brain metastasis.
Noteworthily, the median OS in this work was 15.8 months (95% CI: 10.23–21.37), which was in line with the studies regarding anlotinib joint with PD-1 inhibitors among advanced or metastatic NSCLC (median OS ranged from 10.5 months to 17.3 months) that were reported recently.18,23,31 However, it seemed that OS in this work was longer than the median OS of anlotinib or immunotherapy single agent (median OS was 9.6 and approximately 12.5 months, respectively).15,42 The potential explanation might be the continuous approval of targeted drugs and immunotherapy since 2018. Both targeted drugs (third-generation EGFR-TKIs and promising targeted drugs) and other immunotherapies were useful for advanced or metastatic NSCLC when anlotinib plus PD-1 inhibitors regimen was ineffective.43
The overall adverse reactions were acceptable, which was in accordance with the toxicity landscape in previous work.31 Noteworthily, the prevalence of grade 3–4 adverse reactions was 41.1%, which was slightly higher than the toxicity profile of anlotinib monotherapy or pembrolizumab monotherapy among NSCLC patients (adverse reaction with grade 3–4 was around 39% and 16%, respectively).15,42 Still and all, toxicity landscape of anlotinib plus PD-1 inhibitors was tolerable for advanced or metastatic NSCLC in view of the fact that no death-related toxicity was detected. Additionally, anlotinib caused hypertension, hand-foot syndrome, and proteinuria. Other specific toxicities, such as rash, hepatotoxicity, RCCEP, and pneumonia, might result from PD-1 inhibitors treatment.44 Notably, RCCEP might be a specific toxicity of camrelizumab. Therefore, the real incidence for RCCEP of camrelizumab was 21.7%.45 In a word, the administration of anlotinib plus PD-1 inhibitors achieved tolerable toxicity landscape.
From the objective view, our study had unavoidable limitations in three aspects: firstly, small sample size, four PD-1 inhibitors introduced in the present study and deficiency of PD-L1 expression data helped to compromise the conclusion in terms of feasibility and safety of anlotinib plus PD-1 inhibitors. Another limitation of this study was the absence of comprehensive genetic profiling using NGS or PCR methods, which might provide a more detailed understanding of the genetic factors contributing to treatment response. Future prospective studies should incorporate these techniques to explore a wider range of genetic mutations, offering in-depth insights into predictive markers of therapy efficacy for patients with NSCLC when received anlotinib plus PD-1 inhibitors in clinical practice. Even so, the present study was of promising clinical implications to highlight real-world medical evidence for anlotinib combined with PD-1 inhibitors in previously treated advanced or metastatic NSCLC.
Conclusion
Anlotinib plus PD-1 inhibitors exhibited tolerable toxicity and enhanced anticancer activity in previously treated patients with advanced NSCLC in clinical practice. This conclusion should be validated in future, prospective clinical trials.
Disclosure
The authors declare that there are no conflicts of interest.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Xu K, Zhang C, Du T, et al. Progress of exosomes in the diagnosis and treatment of lung cancer. Biomed Pharmacother. 2021;134(111111):111111. doi: 10.1016/j.biopha.2020.111111 [DOI] [PubMed] [Google Scholar]
- 3.Massafra M, Passalacqua MI, Gebbia V, et al. Immunotherapeutic Advances for NSCLC. Biologics. 2021;15:399–417. doi: 10.2147/btt.s295406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun J, Dong M, Xiang X, Zhang S, Wen D. Notch signaling and targeted therapy in non-small cell lung cancer. Cancer Lett. 2024;585(216647):216647. doi: 10.1016/j.canlet.2024.216647 [DOI] [PubMed] [Google Scholar]
- 5.Remon J, Steuer CE, Ramalingam SS, Felip E. Osimertinib and other third-generation EGFR TKI in EGFR-mutant NSCLC patients. Ann Oncol. 2018;29(suppl_1):i20–i27. doi: 10.1093/annonc/mdx704 [DOI] [PubMed] [Google Scholar]
- 6.Niu M, Yi M, Li N, Luo S, Wu K. Predictive biomarkers of anti-PD-1/PD-L1 therapy in NSCLC. Exp Hematol Oncol. 2021;10(1):18. doi: 10.1186/s40164-021-00211-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu J, Yuan Y, Wan X, et al. Immunotherapy (excluding checkpoint inhibitors) for stage I to III non-small cell lung cancer treated with surgery or radiotherapy with curative intent. Cochrane Database Syst Rev. 2021;12(12):Cd011300. doi: 10.1002/14651858.CD011300.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Zeng L, Li Y, et al. Anlotinib combined with PD-1 blockade for the treatment of lung cancer: a real-world retrospective study in China. Cancer Immunol Immunother. 2021;70(9):2517–2528. doi: 10.1007/s00262-021-02869-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Yang Y, Yu J, et al. Efficacy and safety of anti-PD-1/PD-L1 in combination with chemotherapy or not as first-line treatment for advanced non-small cell lung cancer: a systematic review and network meta-analysis. Thorac Cancer. 2022;13(3):322–337. doi: 10.1111/1759-7714.14244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Q, Wang W, Yuan Q, et al. Correlation between immune-related adverse events and the efficacy of PD-1/PD-L1 inhibitors in the treatment of non-small cell lung cancer: systematic review and meta-analysis. Cancer Chemother Pharmacol. 2022;89(1):1–9. doi: 10.1007/s00280-021-04375-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shukla NA, Yan MN, Hanna N. The story of angiogenesis inhibitors in non-small-cell lung cancer: the past, present, and future. Clin Lung Cancer. 2020;21(4):308–313. doi: 10.1016/j.cllc.2020.02.024 [DOI] [PubMed] [Google Scholar]
- 12.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi: 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa K, Garon EB, Seto T, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet Oncol. 2019;20(12):1655–1669. doi: 10.1016/s1470-2045(19)30634-5 [DOI] [PubMed] [Google Scholar]
- 14.Xie C, Wan X, Quan H, et al. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci. 2018;109(4):1207–1219. doi: 10.1111/cas.13536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han B, Li K, Wang Q, et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol. 2018;4(11):1569–1575. doi: 10.1001/jamaoncol.2018.3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villaruz LC, Socinski MA. The role of anti-angiogenesis in non-small-cell lung cancer: an update. Curr Oncol Rep. 2015;17(6):26. doi: 10.1007/s11912-015-0448-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jayson GC, Kerbel R, Ellis LM, Harris AL. Antiangiogenic therapy in oncology: current status and future directions. Lancet. 2016;388(10043):518–529. doi: 10.1016/s0140-6736(15)01088-0 [DOI] [PubMed] [Google Scholar]
- 18.Wang P, Fang X, Yin T, et al. Efficacy and safety of Anti-PD-1 plus anlotinib in patients with advanced non-small-cell lung cancer after previous systemic treatment failure-A retrospective study. Front Oncol. 2021;11(628124). doi: 10.3389/fonc.2021.628124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin R, Liu C, Zheng S, et al. Molecular heterogeneity of anti-PD-1/PD-L1 immunotherapy efficacy is correlated with tumor immune microenvironment in East Asian patients with non-small cell lung cancer. Cancer Biol Med. 2020;17(3):768–781. doi: 10.20892/j.issn.2095-3941.2020.0121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrelli F, Ferrara R, Signorelli D, et al. Immune checkpoint inhibitors and chemotherapy in first-line NSCLC: a meta-analysis. Immunotherapy. 2021;13(7):621–631. doi: 10.2217/imt-2020-0224 [DOI] [PubMed] [Google Scholar]
- 21.Sun X, Xu J, Xie L, Guo W. Effectiveness and tolerability of Anlotinib Plus PD-1 inhibitors for patients with previously treated metastatic soft-tissue sarcoma. Int J Gen Med. 2022;15(7581–7591. doi: 10.2147/ijgm.s379269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, Li L, Jiang Z, Wang B, Pan Z. Anlotinib optimizes anti-tumor innate immunity to potentiate the therapeutic effect of PD-1 blockade in lung cancer. Cancer Immunol Immunother. 2020;69(12):2523–2532. doi: 10.1007/s00262-020-02641-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhai C, Zhang X, Ren L, et al. The efficacy and safety of anlotinib combined with PD-1 antibody for third-line or further-line treatment of patients with advanced non-small-cell lung cancer. Front Oncol. 2020;10(619010). doi: 10.3389/fonc.2020.619010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 25.Hao YY, Qiao YP, Cheng JD. Clinical activity and safety of anlotinib combined with PD-1 blockades for patients with previously treated small cell lung cancer. Int J Gen Med. 2021;14(10483–10493. doi: 10.2147/ijgm.s337316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng JD, Chai LX, Zhao ZP, Hao YY, Li S. Efficacy and safety of anlotinib for patients with advanced NSCLC who progressed after standard regimens and the preliminary analysis of an efficacy predictor. Cancer Manag Res. 2020;12(5641–5650. doi: 10.2147/cmar.s253366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song PF, Xu N, Li Q. Efficacy and safety of anlotinib for elderly patients with previously treated extensive-stage SCLC and the prognostic significance of common adverse reactions. Cancer Manag Res. 2020;12:11133–11143. doi: 10.2147/cmar.s275624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren S, Xiong A, Yu J, et al. Camrelizumab plus famitinib in previously chemo-immunotherapy treated patients with advanced NSCLC: results from an open-label multicenter Phase 2 basket study. Cancer Immunol Immunother. 2024;73(7):124. doi: 10.1007/s00262-024-03715-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brozos-Vázquez EM, Díaz-Peña R, García-González J, et al. Immunotherapy in nonsmall-cell lung cancer: current status and future prospects for liquid biopsy. Cancer Immunol Immunother. 2021;70(5):1177–1188. doi: 10.1007/s00262-020-02752-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulz C, Gandara D, Berardo CG, et al. Comparative efficacy of second- and subsequent-line treatments for metastatic NSCLC: a fractional polynomials network meta-analysis of cancer immunotherapies. Clin Lung Cancer. 2019;20(6):451–460.e455. doi: 10.1016/j.cllc.2019.06.017 [DOI] [PubMed] [Google Scholar]
- 31.Zhang W, Zhang C, Yang S, et al. Immune checkpoint inhibitors plus anlotinib versus anlotinib alone as third-line treatment in advanced non-small-cell lung cancer: a retrospective study. Future Oncol. 2021;17(31):4091–4099. doi: 10.2217/fon-2021-0649 [DOI] [PubMed] [Google Scholar]
- 32.Segal EM. Immunotherapy in the frontline management of advanced and metastatic NSCLC. Am J Manag Care. 2021;27(18 Suppl):S323–s332. doi: 10.37765/ajmc.2021.88769 [DOI] [PubMed] [Google Scholar]
- 33.Wang DD, Shaver LG, Shi FY, et al. Comparative efficacy and safety of PD-1/PD-L1 immunotherapies for non-small cell lung cancer: a network meta-analysis. Eur Rev Med Pharmacol Sci. 2021;25(7):2866–2884. doi: 10.26355/eurrev_202104_25541 [DOI] [PubMed] [Google Scholar]
- 34.Lu S, Wang J, Cheng Y, et al. Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced non-small cell lung cancer: 2-year follow-up from a randomized, open-label, phase 3 study (CheckMate 078). Lung Cancer. 2021;152:7–14. doi: 10.1016/j.lungcan.2020.11.013 [DOI] [PubMed] [Google Scholar]
- 35.Guo F, Cui J. Anti-angiogenesis: opening a new window for immunotherapy. Life Sci. 2020;258(118163):118163. doi: 10.1016/j.lfs.2020.118163 [DOI] [PubMed] [Google Scholar]
- 36.He YY, Zhang XC, Yang JJ, et al. Prognostic significance of genotype and number of metastatic sites in advanced non-small-cell lung cancer. Clin Lung Cancer. 2014;15(6):441–447. doi: 10.1016/j.cllc.2014.06.006 [DOI] [PubMed] [Google Scholar]
- 37.Fathi Z, Syn NL, Zhou JG, Roudi R. Molecular epidemiology of lung cancer in Iran: implications for drug development and cancer prevention. J Hum Genet. 2018;63(7):783–794. doi: 10.1038/s10038-018-0450-y [DOI] [PubMed] [Google Scholar]
- 38.Cavanna L, Citterio C, Orlandi E. Immune checkpoint inhibitors in EGFR-mutation positive TKI-treated patients with advanced non-small-cell lung cancer network meta-analysis. Oncotarget. 2019;10(2):209–215. doi: 10.18632/oncotarget.26541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019;7(5):387–401. doi: 10.1016/s2213-2600(19)30084-0 [DOI] [PubMed] [Google Scholar]
- 40.Nogami N, Barlesi F, Socinski MA, et al. IMpower150 final exploratory analyses for atezolizumab plus bevacizumab and chemotherapy in key NSCLC patient subgroups with EGFR mutations or metastases in the liver or Brain. J Thorac Oncol. 2022;17(2):309–323. doi: 10.1016/j.jtho.2021.09.014 [DOI] [PubMed] [Google Scholar]
- 41.Liang L, Hui K, Hu C, et al. Autophagy inhibition potentiates the anti-angiogenic property of multikinase inhibitor anlotinib through JAK2/STAT3/VEGFA signaling in non-small cell lung cancer cells. J Exp Clin Cancer Res. 2019;38(1):71. doi: 10.1186/s13046-019-1093-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/s0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 43.Ye Z, Huang Y, Ke J, et al. Breakthrough in targeted therapy for non-small cell lung cancer. Biomed Pharmacother. 2021;133(111079):111079. doi: 10.1016/j.biopha.2020.111079 [DOI] [PubMed] [Google Scholar]
- 44.Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–2471. doi: 10.1016/s0140-6736(17)31827-5 [DOI] [PubMed] [Google Scholar]
- 45.Wang F, Qin S, Sun X, et al. Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: data derived from a multicenter phase 2 trial. J Hematol Oncol. 2020;13(1):47. doi: 10.1186/s13045-020-00886-2 [DOI] [PMC free article] [PubMed] [Google Scholar]