Abstract
Blue-green eggs exhibit unique shell color; however, compared to commercial layers, blue-green eggshell chickens have lower egg production and lack uniform shell colors. Aiming to confirm the molecular mechanisms that affect shell color and egg production, this study collected the uteruses of 12 blue-green eggshell chickens (BG group) and six Hy-Line layers (Brown group), which had significantly different shell color indexes (SCI) and egg numbers at 300 days of age (EN300). Transcriptome sequencing and comparative analyses were subsequently performed. BG hens were divided into two groups for comparative analysis (BGblue vs. BGgreen and BGlow vs. BGhigh, respectively), based on the differences in SCI and EN300, respectively. The result of weighted gene co-expression network (WGCNA) analysis showed that the sequenced and mapped 16,785 genes were clustered into 18 modules, among which six modules with a total of 4270 genes were highly correlated with SCI and EN300 traits. Five hundred and eleven differentially expressed genes (DEGs) belonged to the six key modules. Through KEGG mapping, GO enrichment, and Cytoscape network analysis, nine Hub genes were tightly associated with SCI and EN300. The up-regulated genes were CCR2, CCR8, CD40LG, IL18RAP, INHBA, and P2RY13, while the down-regulated genes were ABCA13, ADCY2, and GRM1. Co-analyses with the results of comparisons between the BG subgroups revealed that the expression of solute carrier (SLC) proteins and ABC transporters were highly related to eggshell color, while cytokine-cytokine receptor interactions and neuroactive ligand-receptor interactions were key pathways affecting egg production. The expression of extracellular cytokines and membrane receptors were significantly up-regulated in low-yield chickens. The candidate DEGs and pathways found in the study will assist in clarifying the molecular mechanisms affecting shell color and egg production, and improve the breeding of blue-green eggshell chickens.
Keywords: Blue-green eggshell chicken, Shell color, Egg production, Transcriptome, Uterus
Introduction
Egg is one of the most important sources of animal protein for humans, and global egg production reached eighty-seven million tons in 2022 (https://www.fao.org/faostat/zh/#data/QCL). The main colors of eggshell are brown, white, pink, and blue-green, among which the blue- and green-shelled eggs display a unique blue-green color and occupy certain markets in Southeast Asian countries (Guyonnet, 2022). Compared to the high egg production of layers after long-term breeding (e.g., White Leghorn, Hy-Line brown, Lohmann pink), blue-green eggshell chickens usually have lower production performances (i.e., laying number, clutch length, egg weight, etc.) and multiple outlook traits (i.e., shell color, skin color, feather color, etc.). Liao et al. (2016) found that the cumulative egg number of Dongxiang blue eggshell chicken at 25–45 weeks of age was 99.19, which was significantly lower than that of White Leghorn (= 124.42) at the same period. Dalirsefat (2015) and Xu (2018b) reported that there were different subtypes of shell color in many blue-green eggshell chicken breeds. For shell color, the objective test is to measure its L*, a*, b* values using a colorimeter, indicating the color lightness (value between 0 = black and 100 = white) and “position” on the red-green scale (<0 = green; >0 = red) and on the blue-yellow scale (<0 = blue; >0 = yellow), respectively (Cavero et al., 2012; Zeng et al., 2022). Studies also found that the shell color index (SCI), equaling L* minus a* minus b* (L*-a*-b*), can be a criterion for eggshell color breeding. For example, Cavero et al. (2012) reported that commercial brown layers were bred to have dark-brown shells for many years by using the SCI value; the lower the SCI, the darker the shell color. Wang et al. (2023a) reported that green-shelled eggs could be selected using SCI ≈ 75 and/or L* ≈ 80 and/or b* ≈ 12.
Egg formation begins with the development of follicles in the ovary. After the mature follicle ovulates, it passes through the oviduct, and the yolk is enveloped by secreted albumen. Subsequently, the incomplete egg reaches the uterus (i.e., shell gland), and the eggshell is formed from secreted calcium and pigments (Kaspers, 2016). Brown eggshells are caused by the deposition of protoporphyrin-IX, whereas blue-green coloration is mainly caused by biliverdin (Lang and Wells, 1987; Zhao et al., 2006). Studies have demonstrated that the hypothalamic-pituitary-ovarian (HPO) axis directly and powerfully affects the performance of egg production. The axis plays an important role in follicle maturation and ovulation through the stepwise transmission and amplification effects of hormones (Brady et al., 2020; Zhao et al., 2023). In contrast to the numerous scientific reports on the HPO axis (Mishra et al., 2020; Wu et al., 2020; Huang et al., 2022; Wang et al., 2023; Ren et al., 2022; Bhavana et al., 2022), studies in the uterus are less frequent (Yang et al., 2022; Chen et al., 2021). Regarding the molecular mechanisms affecting shell colors, Wang et al. (2013) and Wragg et al. (2013) found that the SLCO1B3 gene was the major-effect gene for blue-green eggshells. In populations of blue-green eggshell chickens such as Dongxiang, Araucana, and Maphche, the SLCO1B3 genes all owned insertion fragments of endogenous avian retrovirus-HP (EAV-HP). Some studies reported other genes related to brown and blue-green eggshells, including ALAS1, ABCG2, ABCA12 (Yang et al., 2022; Zi et al., 2023), however, additional studies are necessary.
The breeding of blue-green eggshell chickens requires further studies on shell color and egg production. The chicken line coded “BG”, originating from the segregating progeny of the cross between two Chinese indigenous breeds, Dongxiang blue eggshell chicken and Jiangshan black-bone chicken, has been bred for black carcasses and blue-green eggs (Wang et al., 2021; 2022; 2023a). After years of breeding, the shell color within the population still exhibits inconsistency, and egg production needs to be improved. This study compared the phenotypic and transcriptome differences between the BG line and a high-yield layer, and aimed to clarify the key genes and pathways involved in shell color and egg production, to provide more insight into molecular-assisted breeding of blue-green eggshell chickens.
Materials and methods
Experimental chickens
Twelve blue-green eggshell hens (i.e., BG group) were from the BG line bred by Hangzhou Academy of Agricultural Sciences. Six brown eggshell hens (i.e., Brown group) were from the Hy-Line brown layers raised by the Hangzhou Academy of Agricultural Sciences. The Animal Experiment Committee of Hangzhou Academy of Agricultural Sciences approved this study. From day-old chicks to laying hens, all birds of the BG line and Hy-Line brown layer were reared following the same management manual and animal welfare requirements of Hy-Line International (https://www.hyline.com/). After the age of 105 days, the chickens were moved into individual cages, fed a laying-period diet. When the hens started laying eggs, day length was increased artificially, in 30 min weekly increments, to 16 h of light per day.
Trait measurement and sample collection
After the first egg was laid, the number of daily eggs from each hen of the BG line and Hy-Line brown layer was recorded daily until the age of 300 days. The total egg number for each hen was referred to as EN300. Around the age of 300 days, the eggs from each chicken were collected, and their shell L*, a*, and b* values were measured using a Minolta Colorimeter CR400 (KonicaMinolta, Tokyo, Japan). The mean SCI value (SCI = L*-a*-b*) of each hen was obtained as its shell color index. In the BG line, the mean and standard deviation (SD) of EN300 (n = 453) were 85.6 and 26.7, whereas those of SCI (n = 398) were 78.75 and 7.61 (Wang et al., 2023a). In the Hy-Line brown layers, the mean and SD of EN300 (n = 65) were 149.1 and 8.7, whereas those of SCI (n = 63) were 8.50 and 2.14. Six hens of the Hy-Line brown layer were randomly chosen as the Brown group. Considering both the egg production and eggshell color traits, 12 hens of the BG line were aimly chosen as the BG group. They could be divided into two subgroups, including six low-production chickens (i.e., BGlow subgroup) and six high-production chickens (i.e., BGhigh subgroup), based on the criterion that the difference of the mean EN300 values between subgroups was larger than the SD value of EN300 in the BG line (i.e., > 26.7). Meanwhile they could be divided into two subgroups, including six blue-eggshell chickens (i.e., BGblue subgroup) and six green-eggshell chickens (i.e., BGgreen subgroup), based on the criterion that the difference of the mean SCI values between subgroups was larger than the SD value of SCI in the BG line (i.e., > 7.61). The trait values for all groups and subgroups are visually shown in Fig. 1. After completing the measurements, all 18 experimental hens were bled to death, and their uterus samples were collected and stored in the liquid nitrogen.
Fig. 1.
Bar plot of the SCI and EN300 values of the groups. (A) The mean and standard deviation of SCI and EN300 traits in the BG group and Brown group. (B) The mean and standard deviation of SCI and EN300 traits in subgroups of the BGblue and BGgreen, and in subgroups of the BGlow and BGhigh.
BG: the BG group; Brown: the Brown group; BGblue: the blue egghell subgroup of the BG group; BGgreen: the green eggshell subgroup of the BG group; BGlow: the low egg production subgroup of the BG group; BGhigh: the high egg production subgroup of the BG group; SCI: shell color index; EN300: egg number until the age of 300 days.
⁎⁎ Double-asterisks between groups represent highly-significant differences (p < 0.01). ⁎⁎⁎ Triple-asterisks between groups represent extremely highly-significant differences (p < 0.001).
RNA Extraction
Total RNA was extracted from uterus samples with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions. The concentration, purity and integrity of RNA was measured using a NanoDrop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA) and an Agilent 2100 Bioanalyzer (Agilent Biotechnologies, Palo Alto, CA, USA). Qualified RNA samples with the concentration ≥ 30 ng/μL and the RNA intergrity number > 8.0 were ensured for the subsequent analyses.
cDNA Library construction and RNA sequencing
cDNA libraries were constructed by using the TruSeq RNA Sample Preparation Kit (Illumina, San Diego, CA, USA) according to the manufacturer's protocol. Briefly, mRNA was enriched using poly-T oligo-attached magnetic beads. After random fragmentation under elevated temperatures in a proprietary Illumina fragmentation buffer, first strand cDNA was synthesized using random oligonucleotides. Second strand cDNA synthesis was subsequently performed using DNA polymerase I and RNase H. The double-stranded cDNA was purified using the AMPure XP system (Beckman Coulter Inc., Indianapolis, IN, USA) and eluted for end repair and poly (A) addition. Sequencing adapters were ligated to the 5′ and 3′ ends of the fragments. Finally, fragments were purified using the AMPure XP system and enriched by PCR amplification to create the cDNA library. The quantification and qualification of the sample library was controlled using the Agilent 2100 Bioanalyzer. Libraries were sequenced using an Illumina NovaSeq 6000 (Illumina, San Diego, CA, USA) at Beijing Tsingke Biotechnology Co. Ltd (Beijing, China) and 150-bp paired-end reads were produced. RNA-seq raw reads in this study were deposited in the NCBI Sequence Read Archive (SRA) and can be accessed from PRJNA1150329 BioProject.
Sequencing data quality-control and map
Using the Fastx toolkit 0.0.14 (http://hannonlab.cshl.edu/fastx_toolkit/), the raw sequencing reads were filtered to obtain clean reads, by removing reads containing adapters or poly-N, and reads with low-quality (e.g., Q-score ≤ 10). The clean reads were subsequently mapped against the chicken reference genome (RefSeq: GCF_016699485.2) from the NCBI website using the sequence alignment program, HISAT2 2.0.5 (Pertea et al., 2016). The mapped reads were then assembled and quantified using StringTie 1.3.0 (Pertea et al., 2016).
RNA-Seq data analyses
WGCNA analysis
Weighted gene co-expression network analysis was performed using the WGCNA package (Langfelder and Horvath, 2008) in R software (version 4.3.1). Briefly, the normalized count values of a total of 16,785 mapped genes from each sample, and the SCI and EN300 values of each sample were input as the matrixes. The best power was set to 16 after optimization. The gene network was constructed using the blockwiseModules function with the following parameters: “minModuleSize = 30, pamRespectDendro = FALSE, and mergeCutHeight = 0.25”. The co-expression modules were detected by using hierarchical clustering (Bordini et al., 2021). After the clusters of genes with similar expression patterns (i.e., modules) were merged, the association values between the expression value of each module (i.e., module eigengene; ME) and the value of the trait were calculated using Pearson's correlation (Bordini et al., 2021).
Differentially expressed genes (DEGs) analysis
Using DESeq2 package (Love et al., 2014) in R software with a criteria that the fold-change of count values must be higher than 2 or lower than −2 (i.e., |log2FC| > 1) and its false discovery rate (FDR) adjusted p-value must be lower than 0.05 (i.e., -log10PValue > 1.3), differential analyses were performed to three comparisons (i.e., BG vs. Brown groups, BGblue vs. BGgreen groups, and BGlow vs. BGhigh groups) and the DEGs sets of each comparison were obtained, respectively (see supplementary Table S1). If the expression of DEGs in the former group was higher than that in the latter group, it was considered an up-regulated gene, whereas it was considered a down-regulated gene if the expression pattern was reversed.
Functional enrichment analysis and visualization
According to the Entrez Gene ID of the DEGs obtained from the comparison of BG vs. Brown, Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) enrichment analyses were performed using the KOBAS website (http://bioinfo.org/kobas/genelist/), and the difference between KEGG pathways or between GO terms was considered significant when their corrected p-value (qvalue) was less than 0.05 (see supplementary Table S2). For the key genes belonging to the seven focused KEGG pathways from the top 15 pathways, visualization and Analyze Network function were performed using Cytoscape 3.10.1 software (Shannon et al., 2003). In further, the KEGG analysis was respectively performed to the DEGs obtained from the comparisons of BGblue vs. BGgreen and BGlow vs. BGhigh.
qRT-PCR validation
To verify the accuracy and repeatability of the RNA-Seq results, 11 DEGs including nine DEGs selected from the above KEGG/GO/Cytoscape analyses and two DEGs which belonged to the most significantly regulated genes in the comparison of BG vs. Brown, were chosen as target validation genes, on which quantitative real-time reverse transcriptase PCR (qRT-PCR) was performed using the same 18 uterus samples. Primers for those genes were designed (Table 1) using the Primer-BLAST tool on the NCBI website (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) and synthesized at Beijing Tsingke Biotechnology Co. Ltd. (Beijing, China). They all passed the validation that primer-dimers and other non-specific products were not formed, and the sequences of PCR products were confirmed as expected by sequencing. PCR was performed using an ABI 7500 Real-Time PCR instrument (ThermoFisher Scientific Inc., Waltham, MA, USA). The 20 μL reaction included forward and reverse primers (0.4 μL each), 50 ng/μL cDNA template (4 μL), enzyme Mix (10 μL), and ddH2O (5.2 μL). The reaction parameters were: pre-denaturation at 95°C for 30 s, and 40 cycles of denaturation at 95°C for 10 s, annealing at 60°C for 30 s and extension at 72 °C for 30 s. The 2−△△Ct method (Livak and Schmittgen, 2001) was used to calculate the expression level of the target genes, with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a reference gene for expression normalization. The GAPDH gene was validated to be most stable expression after comparing with other two appropriate reference genes, i.e., actin beta (ACTB) and TATA-box binding protein (TBP) genes, by using RefFinder software (https://blooge.cn/RefFinder/) which include Delta CT, BestKeeper, Normfinder, and Genorm methods (Xie et al., 2023). The significance of the differences in expression between groups was calculated using the t-test. A p < 0.05, p < 0.01, and p < 0.001 were considered significant, highly-significant, and extremely highly-significant, respectively.
Table 1.
Primers for quantitative real-time PCR validation.
| Gene | Forward primer (5’-3’) | Reverse primer (5’-3’) | Accession No. | Size /bp |
|---|---|---|---|---|
| GAPDH | CTTATGACCACTGTCCATGC | GAAAGCCATTCCAGTAAGCT | NM_204305.2 | 180 |
| ABCA13 | CACAACGTTTAAGATGCTGAC | TGGACAGTAGCCAATAAGAATG | XM_040696008.1 | 130 |
| ADCY2 | ACGTTATCCTTCTGCACACTC | AACAAAGACACTGAGCCCAT | XM_015282305.4 | 127 |
| CCR2 | CCTTCAGATGCCCTCATAAA | GAATGATAAGCCCAAGGATG | NM_001045835.1 | 70 |
| CCR8 | GCCATCTTAGCATCAGTGC | AAGACCTTCCAGCTATTACCG | NM_001030991.2 | 110 |
| CD40LG | AGTGCTGAAGTGGATGACG | CTTGGTGCAGAAGCTGACT | NM_204733.2 | 127 |
| GRM1 | GCAACTACAAGATCATCACCA | CATCTTAGGAGTGAACATGCA | XM_004935585.5 | 83 |
| IL18RAP | GCCAGATAAAGATGCTACCAA | CATTCCCTTGCAGAGTAACA | XM_040657373.2 | 137 |
| INHBA | TCACTGGGAAACAACTTTATACA | CTTTGCACACAAACATTCACT | NM_001396543.1 | 142 |
| NPR1 | CCACTGTCCATGAGCAAAC | TCTCGAGGCATACCTTCTTC | XM_040656181.2 | 113 |
| P2RY13 | CCGTTCTCATTCTTATGCTTC | CCTTGACCCTTGGTTTCTT | XM_040679499.2 | 108 |
| SLCO1B3 | CATAACTGCAGCTGTCTTGAA | GCTGAACGCATCTGTACATAA | NM_001318449.2 | 190 |
Results
Sequencing data summary
A total of 156.79 Gb of high-quality data was produced from the 18 samples. The minimum clean bases of each sample was 7.44 Gb and the minimum Q30 ratio was 95.67%, while their mean values were 8.71 Gb and 96.35%, respectively (Table 2). After comparing the clean reads of each sample to the reference genome, the mapped ratios were between 93.10% and 96.13% (Table 2), and the mean percentages mapped to intronic, exonic, and intergenic regions were 12.39%, 81.21%, and 6.40%, respectively. Those results indicated that the sequencing yield and data quality were suitable for subsequent analyses.
Table 2.
Quality analysis of the sequencing data
| Sample | Clean reads /bp | Clean bases /bp | GC content /% | Q30 /% | Mapped reads /bp | Mapped ratio /% |
|---|---|---|---|---|---|---|
| BG1 | 26,325,787 | 7,885,450,592 | 45.90 | 96.19 | 50,569,301 | 96.05 |
| BG2 | 29,155,471 | 8,731,544,842 | 45.88 | 96.47 | 56,004,279 | 96.04 |
| BG3 | 25,866,520 | 7,747,770,148 | 45.71 | 96.48 | 49,723,072 | 96.11 |
| BG4 | 29,045,307 | 8,697,262,962 | 46.10 | 96.36 | 55,788,327 | 96.04 |
| BG5 | 24,838,656 | 7,438,830,652 | 45.82 | 96.24 | 47,659,296 | 95.94 |
| BG6 | 25,841,112 | 7,736,054,306 | 45.86 | 96.16 | 49,607,527 | 95.99 |
| BG7 | 26,785,761 | 8,015,722,936 | 45.96 | 96.34 | 49,874,122 | 93.10 |
| BG8 | 26,697,162 | 7,992,580,916 | 45.79 | 96.59 | 51,131,151 | 95.76 |
| BG9 | 29,917,999 | 8,958,950,226 | 45.95 | 96.51 | 57,320,139 | 95.80 |
| BG10 | 46,456,104 | 13,846,160,048 | 46.76 | 95.74 | 88,207,016 | 94.94 |
| BG11 | 27,958,123 | 8,368,650,968 | 46.12 | 96.55 | 53,142,588 | 95.04 |
| BG12 | 28,781,378 | 8,613,593,016 | 45.89 | 96.53 | 54,068,511 | 93.93 |
| Brown1 | 29,794,924 | 8,919,388,262 | 46.14 | 96.41 | 56,890,052 | 95.47 |
| Brown2 | 26,678,560 | 7,985,569,460 | 46.09 | 96.52 | 51,290,263 | 96.13 |
| Brown3 | 27,774,263 | 8,316,664,096 | 45.97 | 95.67 | 53,262,957 | 95.89 |
| Brown4 | 29,657,655 | 8,879,048,942 | 45.97 | 96.38 | 56,864,231 | 95.87 |
| Brown5 | 33,104,898 | 9,906,409,332 | 46.17 | 96.62 | 63,247,632 | 95.53 |
| Brown6 | 29,228,553 | 8,748,747,388 | 46.37 | 96.53 | 55,854,131 | 95.55 |
| Mean | 29,106,013 | 8,710,466,616 | 46.03 | 96.35 | 55,583,589 | 95.51 |
WGCNA analysis
A total of 16,785 mapped genes were clustered into 18 modules using the Topological Overlap Matrix (TOM) function in Weighted Gene Co-expression Network Analysis (WGCNA). Fig. 2 shows the results of gene cluster and module color distribution. Among them, the grey60 and turquoise modules had the minimum and maximum gene numbers (i.e., 48 and 4576), respectively, while the grey, purple, pink, black, green, and brown modules mentioned below had 79, 173, 252, 362, 852, and 2552 genes, respectively.
Fig. 2.
Cluster dendrogram of genes and modules by WGCNA analysis, according to the 16,785 mapped genes and the traits (SCI and EN300) of total 18 experimental chickens. Eighteen modules obtained are represented by different colors in the horizontal bar.
The heatmap (Fig. 3) indicates that the correlations between the Module Eigengenes (MEs) and SCI, and between the MEs and EN300 performed oppositely. Those findings indicate that MEs negatively correlated with EN300 were positively correlated with SCI, and MEs positively correlated with EN300 were negatively correlated with SCI. The green module had the highest correlations with SCI and EN300 (−0.73 and 0.69, respectively), with p-values both less than 0.01. The other modules with high correlations with SCI and EN300 included the purple, pink, brown, grey, and black modules. The correlation values were −0.47 and 0.51, −0.47 and 0.45, 0.33 and −0.56, −0.39 and 0.48, −0.39 and 0.27, for the purple, pink, brown, grey, and black modules, respectively. The top five modules correlated with SCI were green, pink, purple, black, and grey, whereas the top five modules correlated with EN300 were green, brown, purple, grey, and pink. Thus, those six modules (i.e., black, brown, green, grey, pink, and purple) could be considered as the key modules correlated with egg production and eggshell color.
Fig. 3.
Heat map of the correlations between modules and traits (SCI and EN300). Each column represents a trait, and each row exhibits the eigengene of a certain module with the representative color. The corresponding correlation, followed with the p-value in parentheses, are included in each cell. The shade of red or blue in each cell represents the magnitude of the positive or negative correlation, respectively.
SCI: shell color index; EN300: egg number until the age of 300 days.
DEGs in BG vs. Brown
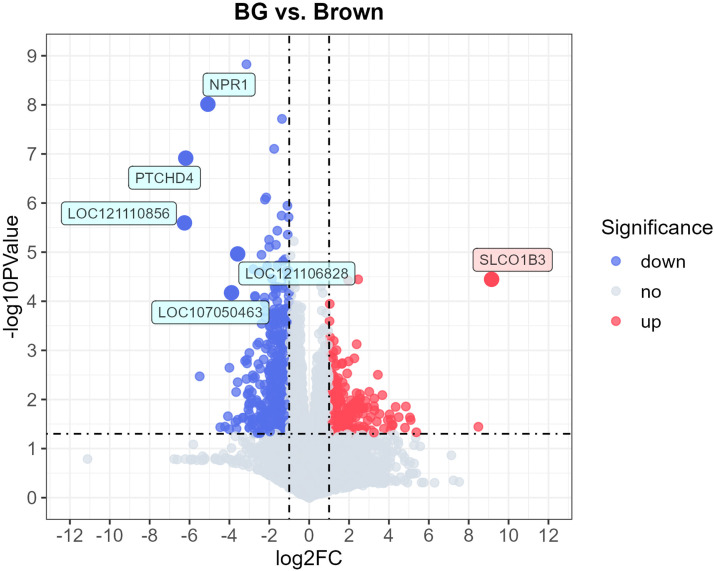
There were 699 DEGs in 16,785 mapped genes, including 264 up-regulated genes and 435 down-regulated genes, from the comparison of BG vs. Brown groups (see supplementary Table S1). Fig. 4 displays the volcano plot of DEGs in BG vs. Brown. The criteria for DEGs were | log2FC | > 1 (i.e., FC > 2 or FC < −2) and -log10PValue > 1.3 (i.e., PValue < 0.05). The DEGs labeled in the plot are the most significant DEGs based on the criteria of | log2FC | > 3.32 (i.e., FC > 10 or FC < −10) and -log10PValue > 3 (i.e., PValue < 0.001), including the up-regulated SLCO1B3 gene and the down-regulated NPR1 and PTCHD4 genes. The information for those three genes is shown in Table 3. Among the 699 DEGs, 511 genes are corresponded to the above-mentioned six WGCNA modules, including 165 up-regulated genes and 346 down-regulated genes with −6.190 < log2FC < 5.090 and 1.306 < -log10PValue < 8.826. However, the SLCO1B3, LOC121110856, and LOC107050463 genes in Table 3 were not contained within the six modules.
Fig. 4.
Volcano plot of DEGs in BG vs. Brown chickens. The two vertical dash lines represent two-fold changes, and the horizontal dash line indicates a p-value less than 0.05. Blue, red, and grey dots represent significantly down-regulated, up-regulated, or non-significantly expressed genes, respectively.
BG: the BG group; Brown: the Brown group; LOC107050463: serine-aspartate repeat-containing protein F-like; LOC121106828: uncharacterized; LOC121110856: uncharacterized; NPR1: natriuretic peptide receptor A; PTCHD4: patched domain containing 4; SLCO1B3: solute carrier organic anion transporter family member 1B3.
Table 3.
The most significantly expressed DEGs in BG vs. Brown chickens.
| Gene | Chromosome | log2FC | -log10PValue | Regulation | Module color | Description |
|---|---|---|---|---|---|---|
| SLCO1B3 | 1 | 9.148 | 4.447 | up | grey | solute carrier organic anion transporter family member 1B3 |
| NPR1 | Z | −5.080 | 8.013 | down | green | natriuretic peptide receptor A |
| PTCHD4 | 3 | −6.190 | 6.915 | down | green | patched domain containing 4 |
| LOC121110856 | 5 | −6.254 | 5.596 | down | grey | uncharacterized |
| LOC121106828 | 14 | −3.585 | 4.964 | down | green | uncharacterized |
| LOC107050463 | – | −3.894 | 4.173 | down | turquoise | serine-aspartate repeat-containing protein F-like |
KEGG and GO analyses in BG vs. Brown
The results of the KEGG pathway analysis for those 511 DEGs in the six key WGCNA modules showed that a total of 106 genes were matched to 79 KEGG pathways (see supplementary Table S2). However, the NPR1, PTCHD4, and LOC121106828 genes listed in Table 3 are not contained within those pathways. Fig. 5 shows the top 15 KEGG pathways sorted by their corrected p-values (qvalue), including 69 genes. Because this study focused on the traits of blue-green eggshells and egg production, the seven pathways that included 49 DEGs are worthy of particular focus, including Cytokine-cytokine receptor interactions, Neuroactive ligand-receptor interactions, Calcium signaling, Vascular smooth muscle contraction, Oocyte meiosis, Primary bile acid biosynthesis, and ABC transporters.
Fig. 5.
The top 15 pathways enriched in KEGG analysis of BG vs. Brown chickens. The X-axis represents the rich factor calculated from the input gene number divided by the background gene number. The Y-axis indicates the KEGG pathways ordered by their corrected p-values (qvalue). The dot colors transmitted from red to green indicate the change in qvalue from low to high, while the dot size indicates the number of input genes.
The results of GO term analysis for those 511 DEGs in the six modules revealed that a total of 1244 GO terms were matched (see supplementary Table S2), including 817 belonging to Biological Process (BP), 181 belonging to Cellular Component (CC), and 246 belonging to Molecular Function (MF). Fig. 6 shows the 32 GO terms sorted by their corrected p-values, which were less than 0.05, including 16 terms in BP, 10 terms in CC, and 6 terms in MF. There were a total of 170 genes in those terms, showing that significant differences in cellular components existed, including in the components of membranes and extracellular space, which mainly involve inflammatory responses, chemokine-mediated signaling, and calcium-mediated signaling.
Fig. 6.
GO enrichment terms of BG vs. Brown chickens, selected according to their corrected p-values (qvalue < 0.05). The X-axis represents the number of input genes. The Y-axis indicates the selected pathways belonging to biological process, cellular component, and molecular function, represented by different colors.
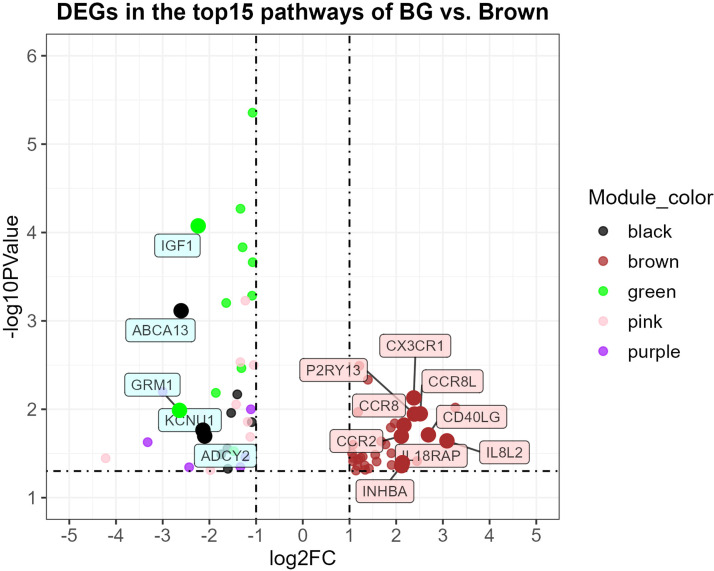
Key DEGs and their Cytoscape network analysis in BG vs. Brown
Fig. 7 displays the volcano plot for 69 DEGs in the top 15 KEGG pathways which were obtained from BG vs. Brown groups. The dot colors represent different WGCNA modules, including the black, brown, green, pink, and purple modules. The results show that all the up-regulated DEGs belonged to the brown module, with a maximum log2FC = 3.267 and maximum -log10PValue = 2.491, whereas the down-regulated DEGs belonged to multiple modules, with minimum log2FC = −4.222 and maximum -log10PValue = 5.356. According to the sorted | log2FC | values, which indicate the differences in gene expression in the BG group compared with the Brown group, the up-regulated genes with high | log2FC | were IL8L2, CD40LG, CCR8L, P2RY13, CX3CR1, CCR8, IL18RAP, INHBA, and CCR2, while the down-regulated genes with high | log2FC | were GRM1, ABCA13, IGF1, KCNU1, and ADCY2.
Fig. 7.
Volcano plot of differentially expressed genes (DEGs) that were in the top 15 KEGG pathways of BG vs. Brown chickens. The two vertical dashed lines represent two-fold changes, while the horizontal dashed line indicates an adjusted p-value less than 0.05. Black, brown, green, pink, and purple dots represent the DEGs belonging to the module with the corresponding color.
BG: the BG group; Brown: the Brown group; ABCA13: ATP binding cassette subfamily A member 13; ADCY2: adenylate cyclase 2; CCR2: C-C motif chemokine receptor 2; CCR8: C-C motif chemokine receptor 8; CCR8L: C-C chemokine receptor 8 like; CD40LG: CD40 ligand; CX3CR1: C-X3-C motif chemokine receptor 1; GRM1: glutamate metabotropic receptor 1; IGF1: insulin like growth factor 1; IL18RAP: interleukin 18 receptor accessory protein; IL8L2: interleukin 8-like 2; INHBA: inhibin beta A subunit; KCNU1: potassium calcium-activated channel subfamily U member 1; P2RY13: purinergic receptor P2Y13.
Fig. 8 displays the cytoscape network generated based on the 49 DEGs in seven focused KEGG pathways which were obtained from BG vs. Brown groups. After excluding 16 genes that were not part of any network, 33 genes formed two major networks, i.e., up-regulated and down-regulated networks which located at the right-side and left-side of Fig. 8, respectively. All of the genes in the up-regulated network belonged to the brown module, whereas those in the down-regulated network belonged to the black and green modules. Considering the correlation results between modules and traits (Fig. 3), the genes in the up-regulated network had high negative correlations with EN300, indicating that higher expressions of those genes were associated with lower egg production, whereas the genes in down-regulated network had high negative correlations with SCI, and thus, indicating that higher expressions of those genes were associated with a lower shell color index. Based on the degree and weight of the nodes that indicate a genes’ importance in the network, the genes with high degrees and weights in the up-regulated network included CD40LG, IL12RB2, CCR8, IL18RAP, INHBA, P2RY13, CH25H, and CCR2. The genes with high degrees and weights in the down-regulated network included ADCY2, CHRNA3, ABCA12, GRM1, GRIK2, ABCA13, and PDE1C. Also considering the | log2FC | values of the above-mentioned genes, nine genes were selected as hub genes, including the up-regulated genes, CD40LG, INHBA, IL18RAP, P2RY13, CCR8, and CCR2, and the down-regulated genes, GRM1, ADCY2, and ABCA13.
Fig. 8.
Cytoscape network of differentially expressed genes (DEGs) that belong to the seven key pathways of BG vs. Brown chickens. Each circle represents a gene, the circle colors (i.e., black, brown, and green) indicate the corresponding colors of the modules, the circle size indicates the | log2FC| value, and the blue and red gene names indicate down- and up-regulated genes, respectively. The thickness of the line connecting two circles represents the association weight between the two genes.
BG: the BG group; Brown: the Brown group.
DEGs in the BGblue vs. BGgreen and BGlow vs. BGhigh
Based on comparisons between the BGblue subgroup and BGgreen subgroup, and between the BGlow subgroup and BGhigh subgroup, 21 and 68 DEGs belonging to the six key WGCNA modules were identified, respectively (see supplementary Table S1). The DEGs were matched to 15 and 28 KEGG pathways, respectively, but their corrected p-values were not significant. Fig. 9 shows the four pathways similar to the seven key pathways obtained from the comparison between BG and Brown chickens, including cytokine-cytokine receptor interactions, neuroactive ligand-receptor interactions, calcium signaling, and vascular smooth muscle contraction pathways. The pathways of oocyte meiosis, primary bile acid biosynthesis, and ABC transporters were not found in the comparisons between BG subgroups. Thus, the results indicate that the expression differences in genes between subgroups of the BG line were significantly lower than those between BG and Brown groups.
Fig. 9.
The KEGG pathways chosen based on comparisons of BGblue vs. BGgreen and BGlow vs. BGhigh. The X-axis represents the rich factor calculated from the input gene number divided by the background gene number. The Y-axis represents the selected pathways ordered by the corrected p-value (qvalue). The dot colors from red to green indicate the changes in qvalues from low to high. The dot size indicates the number of input genes.
BGblue: the blue eggshell subgroup of the BG group; BGgreen: the green eggshell subgroup of the BG group; BGlow: the low egg production subgroup of the BG group; BGhigh: the high egg production subgroup of the BG group.
Distribution of DEGs between three comparisons
Table 4 shows the distributions of the DEGs obtained from the three comparisons (i.e., BG vs. Brown, BGblue vs. BGgreen, and BGlow vs. BGhigh) in six key WGCNA modules. The DEGs between BG and Brown chickens were mostly up-regulated genes in the brown module, and mostly down-regulated genes in the other five modules. The number of DEGs from BGlow vs. BGhigh comparisons was significantly lower than that from BG vs. Brown comparisons, but their distributions in the six modules was similar to that of the DEGS in the BG vs. Brown comparisons. Those data indicate that the DEGs in the brown module were likely related to egg production. For example, the IL18RAP gene was significantly up-regulated in both BG vs. Brown and BGlow vs. BGhigh comparisons. The number of DEGs from BGblue vs. BGgreen comparisons was the lowest, and their distribution in the six modules was not similar to that of DEGs from BG vs. Brown comparisons. For example, the PLA2G1B gene in the black module had different expressions in comparisons of BG vs. Brown and BGblue vs. BGgreen, where it was down-regulated and up-regulated, respectively. Those findings indicated that the PLA2G1B gene might not be associated with the shell color index. One reason for this finding is that the phenotypic differences in shell color in the BG line was small, and therefore, the DEGs related to shell color were expressed less. Another reason may be that some DEGs related to shell color could not be matched to KEGG pathways, such as the LOC100859819 and LOC124418118 genes. Such genes were significantly down-regulated in both BG vs. Brown and BGblue vs. BGgreen comparisons, but were not matched to any KEGG pathway.
Table 4.
Summary of differentially expressed genes (DEGs) in comparisons of BG vs. Brown, BGblue vs. BGgreen, and BGlow vs. BGhigh groups.
| Module color | Regulated situation | DEGs in BG vs. Brown | DEGs in BGblue vs. BGgreen | DEGs in BGlow vs. BGhigh | |||||
|---|---|---|---|---|---|---|---|---|---|
| black | up | 0 | 0 | 0 | 0 | 3 | 1 | 6 | 2 |
| down | 49 | 12 | 9 | 8 | 3 | 0 | 7 | 1 | |
| brown | up | 162 | 43 | 34 | 23 | 1 | 1 | 35 | 10 |
| down | 6 | 2 | 0 | 0 | 4 | 1 | 6 | 0 | |
| midnight | up | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| down | 15 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | |
| pink | up | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
| down | 42 | 13 | 9 | 6 | 0 | 0 | 3 | 0 | |
| purple | up | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| down | 41 | 9 | 6 | 4 | 2 | 0 | 2 | 1 | |
| green | up | 3 | 1 | 0 | 0 | 5 | 0 | 1 | 0 |
| down | 193 | 26 | 11 | 8 | 1 | 0 | 5 | 0 | |
| Total | 511a | 106b | 69c | 49d | 21e | 4f | 68g | 15h | |
511 DEGs belonged to the six modules, from a total of 699 DEGs between BG and Brown.
106 DEGs in 79 pathways identified through KEGG analysis of 511 DEGs.
69 DEGs in the top 15 pathways identified through KEGG analysis of 511 DEGs.
49 DEGs in seven key pathways identified through the KEGG analysis of 511 DEGs.
21 DEGs belonged to the six modules from a total of 50 DEGs between BGblue and BGgreen.
Four DEGs in 15 pathways identified through the KEGG analysis of 21 DEGs.
68 DEGs belonged to the six modules from a total of 129 DEGs between BGlow and BGhigh.
15 DEGs in 28 pathways identified through the KEGG analysis of 68 DEGs.
Validation of DEGs in three comparisons
After the aforementioned analyses, the 11 DEGs in Table 1 were selected for validation by qRT-PCR. Fig. 10 displays the mRNA expression levels of the 11 DEGs, as well as their RNA sequencing counts between the BG and Brown groups. Similar comparisons between the BGblue vs. BGgreen and BGlow vs. BGhigh groups are displayed in Supplementary Figure S1. As shown, none of the 11 DEGs were significantly differentially expressed in comparisons between BGblue vs. BGgreen groups. The SLCO1B3 gene was the most significantly up-regulated gene in BG vs. Brown groups, while it was not significantly differentially expressed in comparisons between BGlow vs. BGhigh groups. However, SLCO1B3 expression was marginally higher in BGblue than in BGgreen. The NPR1 gene was the lowest expressed among the significantly down-regulated gene in BG vs. Brown comparisons, but it was significantly higher expressed in BGlow vs. BGhigh comparisons. The ABCA13 and GRM1 genes were significantly down-regulated, whereas the ADCY2 gene was not-significantly down-regulated in BG vs. Brown comparisons. Additionally, CCR2, CCR8, CD40LG, IL18RAP, and P2RY13 genes were significantly up-regulated, whereas the INHBA genes was not-significantly up-regulated in BG vs. Brown group comparisons. They were also not-significantly up-regulated in BGlow vs. BGhigh group comparisons, while the CCR2 gene was significantly up-regulated. The results showed that the differential expression patterns of most genes detected by qRT-PCR were consistent with the observations determined using RNA-seq, thus, confirming that transcriptome sequencing results are reliable, and can be further studied for elucidating the genetic mechanisms of egg production and eggshell color determination.
Fig. 10.
Bar plot of the mRNA expressions of the 11 validated DEGs between the BG and Brown groups, and their count values in RNA-Seq. The gene name is shown in the title of each plot. The left Y-axis indicates the expression level, while the right Y-axis represents the gene counts. The two left histograms of each plot show the means and standard deviations of gene expression in BG and Brown groups, respectively. The two right histograms show the means and standards deviations of the gene counts in the BG and Brown groups, respectively.
BG: the BG group; Brown: the Brown group; ABCA13: ATP binding cassette subfamily A member 13; ADCY2: adenylate cyclase 2; CCR2: C-C motif chemokine receptor 2; CCR8: C-C motif chemokine receptor 8; CD40LG: CD40 ligand; GRM1: glutamate metabotropic receptor 1; IL18RAP: interleukin 18 receptor accessory protein; INHBA: inhibin beta A subunit; NPR1: natriuretic peptide receptor A; P2RY13: purinergic receptor P2Y13; SLCO1B3: solute carrier organic anion transporter family member 1B3.
ns “ns” between groups represent not significant differences (p > 0.05).
* Single-asterisks between groups represent significant differences (p < 0.05).
⁎⁎ Double-asterisks between groups represent highly-significant differences (p < 0.01).
⁎⁎⁎ Triple-asterisks between groups represent extremely highly-significant differences (p < 0.001).
Discussion
Shell color-related genes and their pathways
The main pigment components of eggshell color are protoporphyrin and biliverdin, which are synthesized and secreted in the epithelial cells of the shell glands (Wang et al., 2007; Sparks, 2011). Some genes of solute carrier (SLC) proteins and ABC transporter proteins in the transporter superfamily have been studied for their roles in the synthesis, decomposition, secretion, and transportation of protoporphyrin and biliverdin. Wragg et al. (2013) found that the expression of the SLCO1B3 gene in shell glands and ovaries of blue-green eggshell chickens was higher than that in brown- and white-shelled layers. In Yimeng blue-eggshell chickens, Chen et al. (2020) found that the insertion of the EAV-HP fragment resulted in the high expression of the SLCO1B3 gene in shell glands. Chen and Wang (2022) also reported that the expression of SLCO1B3 in Lueyang black-boned chickens—which lay blue-greenish eggs—was positively correlated with the biliverdin content in shell glands. In the current study, the SLCO1B3 gene was significantly up-regulated in BG chickens compared to Brown layers, highlighting the importance of the SLCO1B3 gene in the formation of blue-green eggshells.
The detection of eggshell pigments revealed that protoporphyrin and biliverdin were expressed simultaneously in blue-green shells (Wang et al., 2009), indicating that the formation of blue-green eggshells is also affected by protoporphyrin. Yang et al. (2022) conducted transcriptome analysis of shell gland samples from White Leghorn, Roland Red light-brown, and Roland Red dark-brown layers. The study found that the ALAS1, SLC25A38, ABCG2, and FLVCR1 genes were associated with protoporphyrin synthesis, and the expression levels of ALAS1 and ABCG2 in the dark-brown shell group were significantly higher than those in the white shell group. Through transcriptome analysis of the shell gland of Youxian ducks, Xu et al. (2018a) found that the differentially expressed genes and their miRNA target genes were associated with ABC transporter and solute carrier family pathways. Comparisons of green eggs to white eggs revealed that gga-miR-144-3p and its target gene ABCG2 were both upregulated. Wang et al. (2023b) also found that ABCG2 expression was lower in the shell gland of blue-green eggshell chickens than that of brown-shell and higher than that of white-shell chickens. Zi et al. (2023) found that ABCA12 and ABCA4 expression in the shell glands of dark blue eggshells from Xuefeng black-bone chickens were higher than those in the light blue eggshells. In the current study, the expression of the ABCG2 gene was down-regulated in the BG group compared to the Brown group, which was consistent with prior reports. Notably, the degree of down-regulation of ABCA13 and ABCA12 genes was stronger than that of ABCG2. Those results suggest that the ABC transporter family may play an important role in the formation of brown eggshells.
Glutamate metabotropic receptor 1 (GRM1) is a G-protein coupled receptor located on the plasma membrane. One of its functions is to cascade the upstream neurotransmitters and the downstream adenylate cyclase, thereby regulating cytosolic calcium ion concentration (Tang et al., 2019). Adenylate cyclase 2 (ADCY2) is a signaling molecule located on the inner side of the plasma membrane. It regulates ATP-cAMP, activating or inhibiting downstream components, and is involved in a wide range of metabolic pathways, such as calcium signaling, oocyte meiosis, and bile secretion (Tang et al., 2019). Zhang et al. (2015) conducted transcriptome analysis on the shell glands of Roland White layers with different shell strengths, and found that DEGs were enriched in calcification-related processes, such as calcium ion transport. Comparisons to chickens with normal eggshell strengths, the expression of the GRM1 gene was found to be down-regulated in chickens with low eggshell strengths. Also, through transcriptome analyses of the liver of Hy-Line brown layers with different eggshell strengths, Han et al. (2023) found that the expression of the ADCY2 gene was down-regulated, while the expression of the SLC7A11 gene was up-regulated in weak eggshells compared to strong eggshells. The current study didn't measure the eggshell strength, however, shell color as well as shell strength, are both traits related to eggshell, and it had been found that the strength of blue-greenish eggshell was significantly lower than that of brown shell (Drabik et al., 2021). In this study, GRM1 and ADCY2 genes were enriched in calcium signaling pathway and were down-regulated in the BG group compared with the Brown group, might proofing that those two genes were related to eggshell traits.
Egg production related genes and their pathways
Molecular studies on egg production performance mainly focus on the hypothalamic-pituitary-ovarian axis. Although ovaries were not evaluated in this study, the uterus is an important organ of the reproductive system. Thus, the comparison results based on uterus samples also reflect the differences in egg performance. The DEGs related to egg production are highly variable in most studies, which requires considerations of the influencing factors, e.g. breeds of the animals. It is also necessary to consider the similarities and differences of pathways, in addition to focusing on individual genes.
From the perspective of inter-breed comparisons, Huang et al. (2022) conducted transcriptome analyses on the ovaries of high-production Ninghai indigenous chickens and the low-production Wuliangshan black-boned chickens. That study found four DEGs related to egg production, including P2RX1, INHBB, VIPR2, and FABP3. The INHBB gene was highly expressed in low-production chickens. The key pathways that DEGs belonged to included calcium signaling, neuroactive ligand-receptor interactions, and cytokine-cytokine receptor interactions. By using RNA-seq of ovarian tissues, Wang et al. (2023) also found that the significant pathways between high-production Lohmann hens and low-production Chengkou mountain chickens included neuroactive ligand-receptor interactions, steroid hormone biosynthesis, and calcium signaling. INHBA, which is the target gene of lncRNA, was highly expressed in low-production chickens. Ren et al. (2022) performed RNA-seq analysis and found that DEGs in ovarian tissues between White Leghorn and Yunnan Dulong chickens were mainly involved in proteolysis, translation, exosomes, and membrane components. Through transcriptome analysis of the follicles of Lohmann brown hens and Jilin black chickens, Chen et al. (2021) identified 18 candidate genes that may be related to egg production. Neuroactive ligand-receptor interactions, cell adhesion molecules, and cAMP signaling pathways may play important roles in influencing follicle development. Similarly in the current study, the INHBA and INHBB genes were up-regulated in the low-production BG chickens compared to the high-production Brown layers. The key pathways also included cytokine-cytokine receptor interactions, neuroactive ligand-receptor interactions, and calcium signaling. However, such comparisons between different breeds must consider the interference of other traits, such as the affection of skin colors and shell colors, i.e., black-boned chickens have melanin deposition, whereas white-skinned chickens do not; some chickens produce white eggs, whereas some chickens produce brown, blue-greenish, or pink eggs. Thus, from the perspective of comparisons within the same breed, Mishra et al. (2020) conducted transcriptome analysis of the pituitary gland and ovaries of high- and low-production Luhua chickens, and found that neuroactive ligands such as FGF23 and LAMB4 were down-regulated in low-production chickens, whereas cytokines such as RELN were up-regulated. Mu et al. (2021) studied the RNA-seq of ovarian samples from high- and low-yielding Changshun green-shell hens, and found that the PRLR, IL15, CCK, and NTRK1 genes were involved in egg production. The neuroactive ligand-receptor interaction pathway and receptor protein kinase may play important roles in regulating ovarian function and egg production. Through hypothalamic, pituitary, and ovarian transcriptomic analyses, Wu et al. (2020) identified 30 candidate genes related to egg laying and the IGF1, NPR1, and ADCY3 genes were down-regulated in low-yielding Xinjiang Yili geese. The key pathways included calcium signaling, gap junctions, focal adhesion, and ECM-receptor interactions. Bhavana et al. (2022) performed RNA-seq of ovary samples and found 28 DEGs related to follicular development and egg laying. Those genes were enriched in pathways such as glutamate receptor activity, immune function, and oocyte maturation. Neuroactive receptors such as GRIA1 and P2RX6 were down-regulated in low-yielding Indian domestic ducks, whereas cytokines such as CCL5 were up-regulated. By using RNA-seq analyses of hypothalamus and ovary, Bello et al. (2021) identified 10 candidate genes that were responsible for egg production, including P2RX1, LPAR2, ADORA1, FN1, AKT3. The expressions of P2RX1, ADCY5, and ADCY8 were down-regulated in low-yielding Muscovy ducks. The pathways involved in egg laying included calcium signaling, ECM-receptor interactions, and focal adhesion. Notably, the candidate genes identified in those studies, as well as the 68 key DEGs obtained from the comparisons (BGlow vs. BGhigh) in the current study, rarely identified the same genes. However, the pathway enrichment analyses in the above-mentioned studies always consistently identified two pathways, i.e., cytokine-cytokine receptor interaction and neuroactive ligand-receptor interaction pathways.
Through RT-PCR detection of cytokine transcripts, Sundaresan et al. (2006) reported that IL-6 and IL-8 in ovaries and IL-1β, IL-6, and TGF-β2 in the oviducts of White Leghorn chickens were up-regulated during the molting period, and significant up-regulation of TGF-β2 in the regressing oviduct might have suppressed leukocyte recruitment thereby preventing the inflammatory response and tissue damage. Sundaresan et al. (2007) also found that the up-regulation of IL-1β, IL-6, IL-10, and TGF-β2 induced cell apoptosis, whereas the up-regulation of chemokines such as chCCLi2 attracted immune cells during postovulatory follicle regression. Using inter- and intra-breed co-analyses, the results of the current study clarified that extracellular cytokines, including interleukins (ILs), chemokines (CCLs), tumor necrosis factor (e.g., INHBA), transforming growth factor (e.g., CD40LG), and corresponding cytokine receptors on the cell membrane (e.g., IL18RAP, CCR2, and CCR8) have significant impacts on egg production. The expressions of those genes were up-regulated in low-yielding chickens, and the mechanism for which may be similar to that suggested by Sundaresan et al. (2006; 2007). Extracellular neuroactive ligands, such as metabolic pheromones, neurotransmitters, endocrine hormones, and corresponding neuroactive receptors on the membrane (e.g. P2RY13) may also have significant impacts on egg production.
Conclusions
By comparing blue-green eggshell chickens (BG chicken line) to high-production brown-shelled layer chickens, and comparing the subgroups in the BG line, it was found that the expression of solute carrier (SLC) proteins and ABC transporters were highly correlated with eggshell color. Cytokine-cytokine receptor interactions and neuroactive ligand-receptor interactions were the two key pathways affecting egg production. This study determined differentially expressed genes related to eggshell color and egg production, including ABCA13, ADCY2, CCR2, CCR8, CD40LG, GRM1, IL18RAP, INHBA, and P2RY13.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in the present study.
Acknowledgments
This work was supported by the project of Agricultural New Breeds from Zhejiang Science and Technology Agency [grant number 2021C02068-9] and the ‘115’ project of Foreign Intelligence Import from Hangzhou Science and Technology Bureau [grant numbers 20230450, 2023; 20220367, 2022].
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2024.104438.
Appendix. Supplementary materials
References
- Bello S.F., Xu H.P., Guo L.J., Li K., Zhen M., Xu Y.B., Zhang S.Y., Bekele E.J., Bahareldin A.A., Zhu W.J., Zhang D.X., Zhang X.Q., Ji C.L., Nie Q.H. Hypothalamic and ovarian transcriptome profiling reveals potential candidate genes in low and high egg production of white Muscovy ducks (Cairina moschata) Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavana K., Foote D.J., Srikanth K., Balakrishnan C.N., Prabhu V.R., Sankaralingam S., Singha H.S., Gopalakrishnan A., Nagarajan M. Comparative transcriptome analysis of Indian domestic duck reveals candidate genes associated with egg production. Sci. Rep. 2022;12:10943. doi: 10.1038/s41598-022-15099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordini M., Zappaterra M., Soglia F., Petracci M., Davoli R. Weighted gene co-expression network analysis identifies molecular pathways and hub genes involved in broiler White Striping and Wooden Breast myopathies. Sci. Rep. 2021;11(1):1776. doi: 10.1038/s41598-021-81303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady K., Porter T.E., Liu H.C., Long J.A. Characterization of the hypothalamo-pituitary-gonadal axis in low and high egg producing turkey hens. Poult. Sci. 2020;99(2):1163–1173. doi: 10.1016/j.psj.2019.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavero D., Schmutz M., Icken W., Preisinger R. Attractive eggshell color as a breeding goal. Lohmann Inf. 2012;47(2):15–21. [Google Scholar]
- Chen J.F., Dalirsefat S.B., Han D.P., Dong X.G., Hua G.Y., Zheng X.T., Xia T.L., Shao T.Q. An EAV-HP insertion in the 5ʹ flanking region of SLCO1B3 is associated with its tissue-expression profile in blue-eggshell Yimeng chickens (Gallus gallus) Poult. Sci. 2020;99(12):6371–6377. doi: 10.1016/j.psj.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Wang Z. A new molecular mechanism supports that blue-greenish egg color evolved independently across chicken breeds. Poult. Sci. 2022;101(12) doi: 10.1016/j.psj.2022.102223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.X., Sun X., Chimbaka I.M., Qin N., Xu X.X., Liswaniso S., Xu R.F., Gonzalez J.M. Transcriptome analysis of ovarian follicles reveals potential pivotal genes associated with increased and decreased rates of chicken egg production. Front. Genet. 2021;12 doi: 10.3389/fgene.2021.622751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalirsefat S.B. China Agricultural University.; Beijing: 2015. Analysis of Eggshell Pigmentation in the Chinese Indigenous Blue-Shelled Chickens. Doctor thesis. [Google Scholar]
- Drabik K., Karwowska M., Wengerska K., Próchniak T., Adamczuk A., Batkowska J. The variability of quality traits of table eggs and eggshell mineral composition depending on hens’ breed and eggshell color. Animals. 2021;11:1204. doi: 10.3390/ani11051204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyonnet V. Does eggshell color really matter? WATT Poult. Int. 2022;61:22–23. [Google Scholar]
- Han G.P., Kim J.H., Kim J.M., Kil D.Y. Transcriptomic analysis of the liver in aged laying hens with different eggshell strength. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Zhang H.Y., Cao H.Y., Zhou W., Xiang X., Yin Z.Z. Transcriptomics and metabolomics analysis of the ovaries of high and low egg production chickens. Animals. 2022;12:2010. doi: 10.3390/ani12162010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspers B. An egg a day-the physiology of egg formation. Lohmann Inf. 2016;50(2):12–17. [Google Scholar]
- Lang M.R., Wells J.W. A review of eggshell pigmentation. World's. Poult. Sci. J. 1987;43:238–246. [Google Scholar]
- Langfelder P., Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinf. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao R., Zhang X., Chen Q., Wang Z., Wang Q., Yang C., Pan Y. Genome-wide association study reveals novel variants for growth and egg traits in Dongxiang blue-shelled and White Leghorn chickens. Anim. Genet. 2016;47(5):588–596. doi: 10.1111/age.12456. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S.K., Chen B., Zhu Q., Xu Z.X., Ning C.Y., Yin H.D., Wang Y., Zhao X.L., Fan X.L., Yang M.Y., Yang D.Y., Ni Q.Y., Li Y., Zhang M.W., Li D.Y. Transcriptome analysis reveals differentially expressed genes associated with high rates of egg production in chicken hypothalamic-pituitary-ovarian axis. Sci. Rep. 2020;10:5976. doi: 10.1038/s41598-020-62886-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu R., Y Y., Yu T.Gegen, Wen D., Wang F., Chen Z., Xu W.B. Transcriptome analysis of ovary tissues from low- and high-yielding Changshun green-shell laying hens. BMC Genomics. 2021;22:349. doi: 10.1186/s12864-021-07688-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertea M., Kim D., Pertea G., Leek J.T., Salzberg S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016;11:1650–1667. doi: 10.1038/nprot.2016.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y., Ding Y.Z., Nong Y.H., Guo A.W., Liu L.L. Comparative analysis of transcriptome levels in ovarian tissues of Yunnan Dulong chicken and White Leghorn chicken. Acta Veterinaria et Zootechnica Sinica. 2022;53(1):43–52. [Google Scholar]
- Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks N.H.C. Eggshell pigments–from formation to deposition. Avian Biol. Res. 2011;4(4):162–167. [Google Scholar]
- Sundaresan N.R., Anish D., Sastry K.V.H., Saxena V.K., Mohan J., Ahmed K.A. Cytokines in reproductive remodeling of molting White Leghorn hens. J Reprod. Immunol. 2006;73(1):39–50. doi: 10.1016/j.jri.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Sundaresan N.R., Saxena V.K., Sastry K.V.H., Nagarajan K. Cytokines and chemokines in postovulatory follicle regression of domestic chicken (Gallus gallus domesticus) Dev. Comp. Immunol. 2007;32(3):253–264. doi: 10.1016/j.dci.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Tang H.M., Finn R.D., Thomas P.D. TreeGrafter: phylogenetic tree-based annotation of proteins with Gene Ontology terms and other annotations. Bioinformatics. 2019;35(3):518–520. doi: 10.1093/bioinformatics/bty625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.H., Cahaner A., Lou L.F., Zhang L., Ge Y., Li Q.H., Zhang X.D. Genetics and breeding of a black-bone and blue eggshell chicken line. 1. Body weight, skin color, and their combined selection. Poult. Sci. 2021;100(5) doi: 10.1016/j.psj.2021.101035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.H., Cahaner A., Lou L.F., Zhang L., Ge Y., Li Q.H., Zhang X.D. Genetics and breeding of a black-bone and blue eggshell chicken line. 2. Laying patterns and egg production in two consecutive generations. Poult. Sci. 2022;101(5) doi: 10.1016/j.psj.2021.101679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.H., Cahaner A., Lou L.F., Zhang L., Ge Y., Li Q.H., Zhang X.D. Genetics and breeding of a black-bone and blue eggshell chicken line. 3. Visual eggshell color and colorimeter parameters in 3 consecutive generations. Poult. Sci. 2023;102(11) doi: 10.1016/j.psj.2023.103052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.H., Ge Y., Zhang L., Wei Y.H., Li Q.H., Zhang X.D., Pan Y.C. The pigments in eggshell with different colour and the pigment regulatory gene expression in corresponding chicken's shell gland. Animal. 2023;17(5) doi: 10.1016/j.animal.2023.100776. [DOI] [PubMed] [Google Scholar]
- Wang X.T., Zhao C.J., Li J.Y., Xu G.Y., Lian L.S., Wu C.X., Deng X.M. Comparison of the total amount of eggshell pigments in Dongxiang brown-shelled eggs and Dongxiang blueshelled eggs. Poult. Sci. 2009;88:1735–1739. doi: 10.3382/ps.2008-00434. [DOI] [PubMed] [Google Scholar]
- Wang X.T., Deng X.M., Zhao C.J., Li J.Y., Xu G.Y., Lian L.S., Wu C.X. Study of the deposition process of eggshell pigments using an improved dissolution method. Poult. Sci. 2007;86:2236–2238. doi: 10.1093/ps/86.10.2236. [DOI] [PubMed] [Google Scholar]
- Wang Z.P., Qu L.J., Yao J.F., Yang X.L., Li G.Q., Zhang Y.Y., Li J.Y., Wang X.T., Bai J.R., Xu G.Y., Deng X.M., Yang N., Wu C.X. An EAV-HP insertion in 5′ flanking region of SLCO1B3 causes blue eggshell in the chicken. PLoS Genetics. 2013;9(1) doi: 10.1371/journal.pgen.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.Y., X G.X.E, Liu C.L., Liu C.B., Song D.J., Li M.H. Comparative transcriptomic profiling in ovarian tissues of Lohmann hens and Chengkou mountain chicken. Front. Biosci. 2023;28(10):267. doi: 10.31083/j.fbl2810267. [DOI] [PubMed] [Google Scholar]
- Wragg D., Mwacharo J.M., Alcalde J.A., Wang C., Han J.L., Gongora J., Gourichon D., Tixier-Boichard M., Hanotte O. Endogenous retrovirus EAV-HP linked to blue egg phenotype in Mapuche fowl. PLoS One. 2013;8(8):e71393. doi: 10.1371/journal.pone.0071393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.P., Zhao X.Y., Chen L., Wang J.H., Duan Y.Q., Li H.Y., Lu L.Z. Transcriptomic analyses of the hypothalamic-pituitary-gonadal axis identify candidate genes related to egg production in Xinjiang Yili geese. Animals. 2020;10(1):90. doi: 10.3390/ani10010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F., Wang J., Zhang B. RefFinder: a web-based tool for comprehensively analyzing and identifying reference genes. Funct. Integr. Genomics. 2023;23:125. doi: 10.1007/s10142-023-01055-7. [DOI] [PubMed] [Google Scholar]
- Xu F.Q., Li A., Lan J.J., Wang Y.M., Yan M.J., Lian S.Y., Wu X. Study of formation of green eggshell color in ducks through global gene expression. PLoS One. 2018;13(1) doi: 10.1371/journal.pone.0191564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.M. Foshan University; Foshan: 2018. Breeding Study of Changshun Blue-Eggshell Chicken. Master thesis. [Google Scholar]
- Yang J., Mao Z.Q., Wang X.Q., Zhuang J.J., Gong S.J., Gao Z.Y., Xu G.Y., Yang N., Sun C.J. Identification of crucial genes and metabolites regulating the eggshell brownness in chicken. BMC Genomics. 2022;23(1):761. doi: 10.1186/s12864-022-08987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L.S., Xu G.Y., Jiang C.Y., Li J.Y., Zheng J.X. Research Note: L*a*b* color space for prediction of eggshell pigment content in differently colored eggs. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J.B., Pan H.B., Liu Y., He Y., Shi H.M., Ge C.R. Interacting networks of the hypothalamic-pituitary-ovarian axis regulate layer hens performance. Genes (Basel) 2023;14(1):141. doi: 10.3390/genes14010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R., Xu G.Y., Liu Z.Z., Li J.Y., Yang N. A study on eggshell pigmentation: biliverdin in blue-shelled chickens. Poult. Sci. 2006;85:546–549. doi: 10.1093/ps/85.3.546. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Zhu F., Liu L., Zheng C.W., Wang D.H., Hou Z.C., Ning Z.H. Integrating transcriptome and genome resequencing data to identify key genes and mutations affecting chicken eggshell qualities. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0125890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zi Q.T., Li P., Lei J.X., Qu X.Y., He C.Q., Yao Y.L., Zou X.Y., Guo S.C. Identification of genes affected blue eggshell coloration in Xuefeng black-bone chickens. Braz. J. Poultry Sci. 2023;26 eRBCA-2022-1729. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.